Search Thermo Fisher Scientific
Thermo Scientific Chemicals
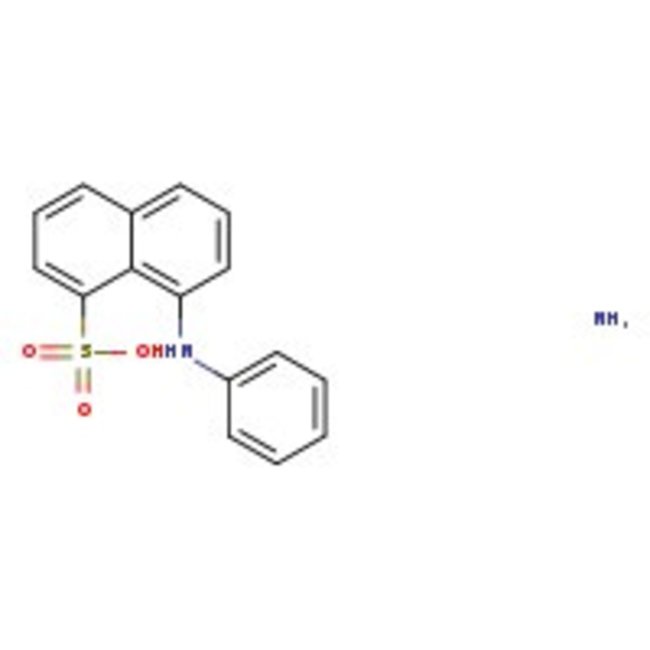
8-Anilinonaphthalene-1-sulfonic acid ammonium salt, 98%
Fluorescent probe for proteins on polyacrylamide gels | CAS: 28836-03-5 | C16H16N2O3S | 316.38 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFJ65538.06 | 5 g |
Catalog number ALFJ65538.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or Material8-Anilinonaphthalene-1-sulfonic acid ammonium salt
CAS28836-03-5
Health Hazard 1H315-H319
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P264b-P280-P302+P352-P305+P351+P338-P332+P313-P362
View more
ANS forms an inclusion complex with cyclodextrin. Such model systems are useful to mimic biological recognition and can be studied by measuring the change in fluorescence of free-ANS to complexed-ANS. When ANS enters the hydrophobic core of cyclodestrin, it’s fluorescence increases. It is commonly used to study molecular assemblies of surfactants and amphiphilic polymers because a blue shift of the emission maximum indicates the fluorophore is located in less polar media.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
ANS forms an inclusion complex with cyclodextrin. Such model systems are useful to mimic biological recognition and can be studied by measuring the change in fluorescence of free-ANS to complexed-ANS. When ANS enters the hydrophobic core of cyclodestrin, it’s fluorescence increases. It is commonly used to study molecular assemblies of surfactants and amphiphilic polymers because a blue shift of the emission maximum indicates the fluorophore is located in less polar media.
Solubility
Soluble in water, 1N NaOH, and methanol.
Notes
Light & Air Sensitive & Hygroscopic. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
ANS forms an inclusion complex with cyclodextrin. Such model systems are useful to mimic biological recognition and can be studied by measuring the change in fluorescence of free-ANS to complexed-ANS. When ANS enters the hydrophobic core of cyclodestrin, it’s fluorescence increases. It is commonly used to study molecular assemblies of surfactants and amphiphilic polymers because a blue shift of the emission maximum indicates the fluorophore is located in less polar media.
Solubility
Soluble in water, 1N NaOH, and methanol.
Notes
Light & Air Sensitive & Hygroscopic. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
Neha Jain.; Mily Bhattacharya.; Samrat Mukhopadhyay. Kinetics of Surfactant-induced Aggregation of Lysozyme Studied by Fluorescence Spectroscopy. Journal of Fluorescence. 2011, 21, (2), 615-625.