Search Thermo Fisher Scientific
Thermo Scientific Chemicals
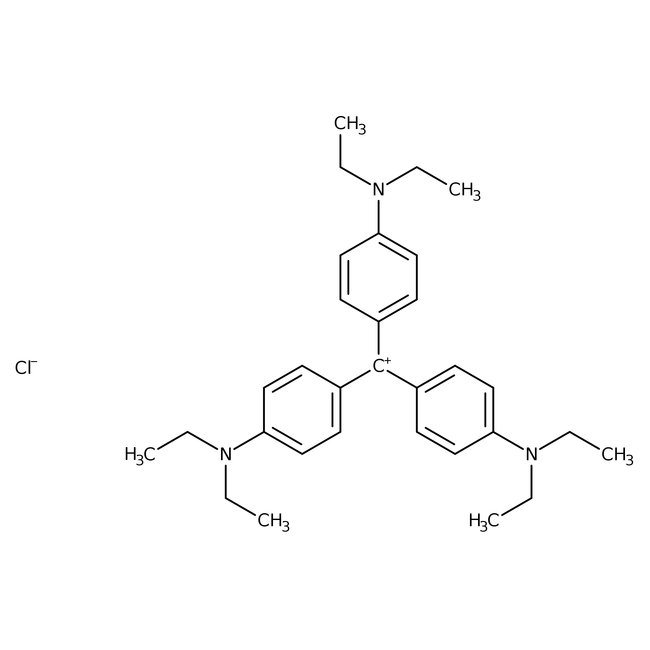
Ethyl Violet
CAS: 2390-59-2 | C31H42ClN3 | 492.15 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFJ63951.22 | 100 g |
Catalog number ALFJ63951.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialEthyl Violet
CAS2390-59-2
Recommended StorageAmbient temperatures
RTECS NumberKH2682000
EINECS Number219-231-5
View more
Ethyl violet has been used to stain pancreatic tissue and elastin in the detection of gram negative bacteria, and as a reagent for the extractive-spectrophotometric determination of anionic surfactants. It has been used in the extraction and spectrophotometric determination of copper. Along with the anionic dye zincon, ethyl violet is used in counterion dye of proteins in SDS-polyacrylamide gel electrophoresis. Along with the anionic dye zincon, ethyl violet is used in counterion dye of proteins in SDS-polyacrylamide gel electrophoresis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl violet has been used to stain pancreatic tissue and elastin in the detection of gram negative bacteria, and as a reagent for the extractive-spectrophotometric determination of anionic surfactants. It has been used in the extraction and spectrophotometric determination of copper. Along with the anionic dye zincon, ethyl violet is used in counterion dye of proteins in SDS-polyacrylamide gel electrophoresis. Along with the anionic dye zincon, ethyl violet is used in counterion dye of proteins in SDS-polyacrylamide gel electrophoresis.
Solubility
Soluble in water (10 mg/ml).
Notes
Dye content approx. 80%
Ethyl violet has been used to stain pancreatic tissue and elastin in the detection of gram negative bacteria, and as a reagent for the extractive-spectrophotometric determination of anionic surfactants. It has been used in the extraction and spectrophotometric determination of copper. Along with the anionic dye zincon, ethyl violet is used in counterion dye of proteins in SDS-polyacrylamide gel electrophoresis. Along with the anionic dye zincon, ethyl violet is used in counterion dye of proteins in SDS-polyacrylamide gel electrophoresis.
Solubility
Soluble in water (10 mg/ml).
Notes
Dye content approx. 80%
RUO – Research Use Only
General References:
- Andrews JJ,; Johnston RV Jr,; Bee DE,; Arens JF. Photodeactivation of ethyl violet: a potential hazard of Sodasorb.. Anesthesiology. 1990, 72(1), 59-64..
- M.M. Martin,; E. Breheret,; F. Nesa,; Y.H. Meyer.Picosecond relaxation path of ethyl violet . Chemical Physics. 1989, 130 (1-3), 279-287.