Search Thermo Fisher Scientific
Thermo Scientific Chemicals
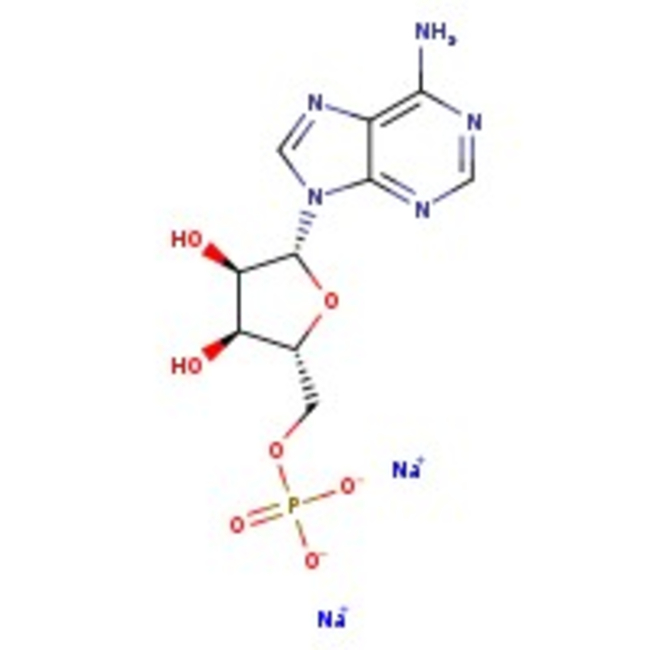
Adenosine-5'-monophosphate disodium salt
CAS: 4578-31-8 | C10H12N5Na2O7P | 391.19 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFJ61643.06 | 5 g |
Catalog number ALFJ61643.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or MaterialAdenosine-5'-monophosphate disodium salt
CAS4578-31-8
ColorWhite
Recommended StorageStore at -20°C
RTECS NumberAU7481600
View more
Adenosine-5'-monophosphate disodium salt is a nucleotide involved in the reactions of cellular energy transfers. It is useful for skin rejuvenation in dermatology and aesthetic dermatology. It acts as an activator of a class of protein kinases called as AMP-activated protein kinase (AMPK). Further, it is used as a substrate for enzymes such as AMP-thymidine kinase, AMP deaminase and 5'-nucleotidase.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Adenosine-5′-monophosphate disodium salt is a nucleotide involved in the reactions of cellular energy transfers. It is useful for skin rejuvenation in dermatology and aesthetic dermatology. It acts as an activator of a class of protein kinases called as AMP-activated protein kinase (AMPK). Further, it is used as a substrate for enzymes such as AMP-thymidine kinase, AMP deaminase and 5′-nucleotidase.
Solubility
Soluble in water, dimethyl sulfoxide and methanol.
Notes
Air and moisture sensitive. Hygroscopic. Incompatible with strong oxidizing agents.
Adenosine-5′-monophosphate disodium salt is a nucleotide involved in the reactions of cellular energy transfers. It is useful for skin rejuvenation in dermatology and aesthetic dermatology. It acts as an activator of a class of protein kinases called as AMP-activated protein kinase (AMPK). Further, it is used as a substrate for enzymes such as AMP-thymidine kinase, AMP deaminase and 5′-nucleotidase.
Solubility
Soluble in water, dimethyl sulfoxide and methanol.
Notes
Air and moisture sensitive. Hygroscopic. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Cade-Menun, B. J. Improved peak identification in 31P-NMR spectra of environmental samples with a standardized method and peak library. Geoderma 2015, 257-258, 102-114.
- Ortiz-Villanueva, E.; Jaumot, J.; Benavente, F.; Piña, B.; Sanz-Nebot, V.; Tauler, R. Combination of CE-MS and advanced chemometric methods for high-throughput metabolic profiling. Electrophoresis 2015, 36 (18), 2324-2335.