Search Thermo Fisher Scientific
Thermo Scientific Chemicals
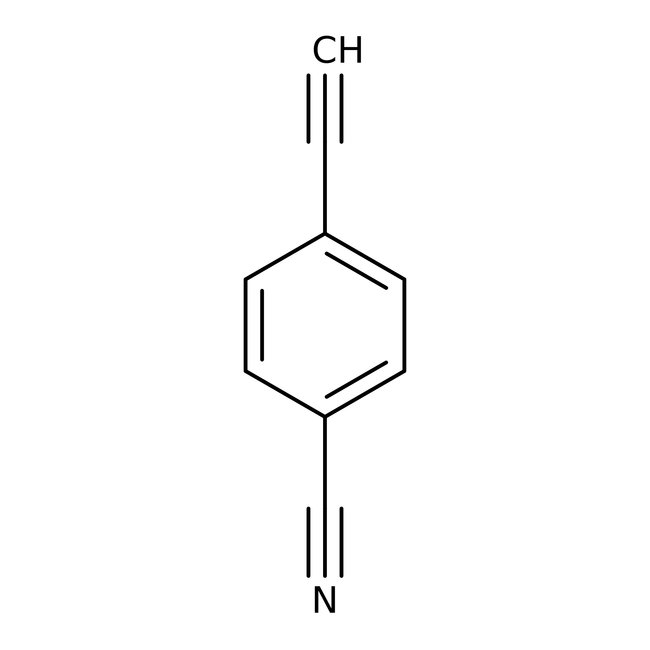
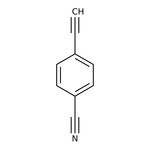
4-Ethynylbenzonitrile, 97%
CAS: 3032-92-6 | C9H5N | 127.146 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFH61244.03 | 1 g |
Catalog number ALFH61244.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Specifications
Chemical Name or Material4-Ethynylbenzonitrile
CAS3032-92-6
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H331-H302-H312-H315-H319-H335
Toxic if inhaled.
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H331-H302-H312-H315-H319-H335
Toxic if inhaled.
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
4-Ethynylbenzonitrile is used as a synthetic fragment and as a test compound for cross-coupling reactions. It is used in the study of hydrogen bond formation in multifunctional molecules due to the presence of four hydrogen bonding sites. It is also involved in the preparation of 4-[(trimethylsilyl)ethynyl]benzonitrile.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Ethynylbenzonitrile is used as a synthetic fragment and as a test compound for cross-coupling reactions. It is used in the study of hydrogen bond formation in multifunctional molecules due to the presence of four hydrogen bonding sites. It is also involved in the preparation of 4-[(trimethylsilyl)ethynyl]benzonitrile.
Solubility
Slightly soluble in water.
Notes
Incompatible with strong oxidizing agents, acids and organic materials.
4-Ethynylbenzonitrile is used as a synthetic fragment and as a test compound for cross-coupling reactions. It is used in the study of hydrogen bond formation in multifunctional molecules due to the presence of four hydrogen bonding sites. It is also involved in the preparation of 4-[(trimethylsilyl)ethynyl]benzonitrile.
Solubility
Slightly soluble in water.
Notes
Incompatible with strong oxidizing agents, acids and organic materials.
RUO – Research Use Only
General References:
- Zhang, C.; Liu, J.; Xia, C. Palladium-N-heterocyclic carbene (NHC)-catalyzed synthesis of 2-ynamides via oxidative aminocarbonylation of alkynes with amines. Catal. Sci. Technol. 2015, 5 (10), 4750-4754.
- Tadeo-León, J.; Fomine, S.; Bizarro, M.; Guadarrama, P. Fully conjugated push-pull dendrons with high dipole moments in excited state; synthesis and theoretical rationalization. J. Phys. Org. Chem. 2015, 28 (4), 304-311.