Search Thermo Fisher Scientific
Thermo Scientific Chemicals
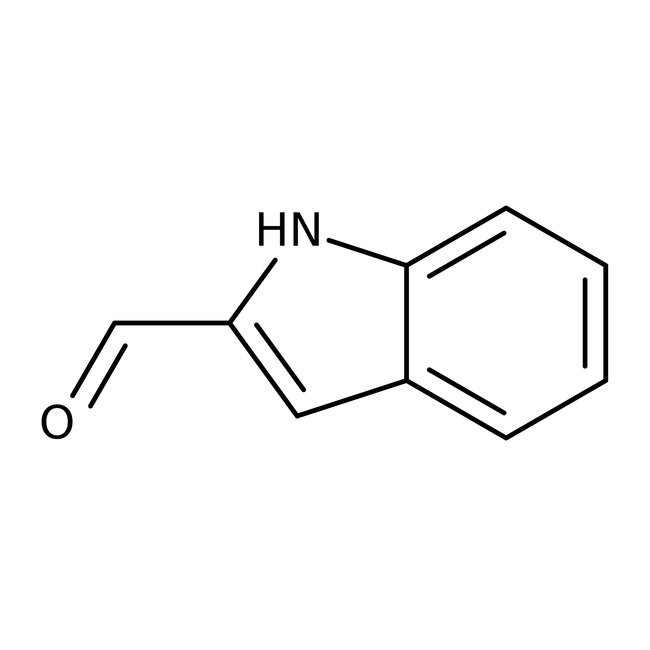
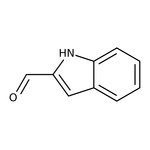
Indole-2-carboxaldehyde, 97%
CAS: 19005-93-7 | C9H7NO | 145.16 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFH54638.03 | 1 g |
Catalog number ALFH54638.03
Price (MYR)
843.00
EA
Quantity:
1 g
Price (MYR)
843.00
EA
Specifications
Chemical Name or MaterialIndole-2-carboxaldehyde
CAS19005-93-7
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H319
Causes serious eye irritation.
H319
Causes serious eye irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
Indole-2-carboxaldehyde is a useful starter in indole and natural product chemistry.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Indole-2-carboxaldehyde is a useful starter in indole and natural product chemistry.
Solubility
Soluble in Methanol.
Notes
Air sensitive. Strong oxidizers and strong reducing agents are incompatible. Store at room temperature.
Indole-2-carboxaldehyde is a useful starter in indole and natural product chemistry.
Solubility
Soluble in Methanol.
Notes
Air sensitive. Strong oxidizers and strong reducing agents are incompatible. Store at room temperature.
RUO – Research Use Only
General References:
- Michael D. Meyer.; Lawrence I. Kruse. Ergoline synthons: Synthesis of 3,4-dihydro-6-methoxybenz[cd]indol-5(1H)-one (6-methoxy-Uhle's ketone) and 3,4-dihydrobenz[cd]indol-5(1H)-one (Uhle's ketone) via a novel decarboxylation of indole-2-carboxylates. J. Org. Chem. 1984, 49 (17),3195-3199.
- Subhasish Biswas.; Virender Singh.; Sanjay Batra. Morita-Baylis-Hillman reaction of indole-2-carboxaldehyde: new vistas for indole-annulated systems. Tetrahedron. 2010, 66 (39),7781-7786.