Search Thermo Fisher Scientific
Thermo Scientific Chemicals
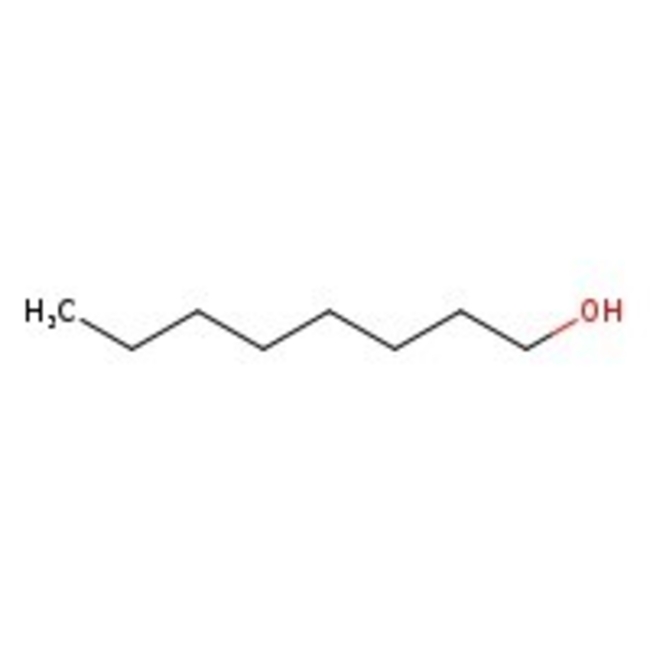
1-Octanol, natural, 98%
CAS: 111-87-5 | C8H18O | 130.23 g/mol
Catalog number ALFH36188.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or Material1-Octanol
Name NoteNatural
CAS111-87-5
Health Hazard 1H227-H319
Health Hazard 2GHS H Statement
H315-H319-H227
Causes skin irritation.
Causes serious eye irritation.
Combustible liquid.
H315-H319-H227
Causes skin irritation.
Causes serious eye irritation.
Combustible liquid.
View more
1-Octanol is a synthetic reagent used to produce esters. It inhibits T-type calcium channels and is used to determine the partition coefficient between compounds and the cytosol. It is used to evaluate the lipophilicity of pharmaceutical products. It is also used as a solvent for protective coatings, waxes, and oils, and as a raw material for plasticizers.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Octanol is a synthetic reagent used to produce esters. It inhibits T-type calcium channels and is used to determine the partition coefficient between compounds and the cytosol. It is used to evaluate the lipophilicity of pharmaceutical products. It is also used as a solvent for protective coatings, waxes, and oils, and as a raw material for plasticizers.
Solubility
Not miscible in water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents, acids, acid chlorides and acid anhydrides.
1-Octanol is a synthetic reagent used to produce esters. It inhibits T-type calcium channels and is used to determine the partition coefficient between compounds and the cytosol. It is used to evaluate the lipophilicity of pharmaceutical products. It is also used as a solvent for protective coatings, waxes, and oils, and as a raw material for plasticizers.
Solubility
Not miscible in water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents, acids, acid chlorides and acid anhydrides.
RUO – Research Use Only
General References:
- Hiromi Yamashita; Yuichi Ichihashi; Masaru Harada; Gina Stewart; Marye Anne Fox; Masakazu Anpo. Photocatalytic degradation of 1-octanol on anchored titanium oxide and on TiO2 powder catalysts. Journal of Catalysis. 1996, 158,(1), 97-101.
- Tai-Horng Young; Liao-Ping Cheng; Dar-Jong Lin; Ling Fane; Wen-Yuan Chuang. Mechanisms of PVDF membrane formation by immersion-precipitation in soft (1-octanol) and harsh (water) nonsolvents. Polymer. 1999, 40,(19), 5315-5323.