Search Thermo Fisher Scientific
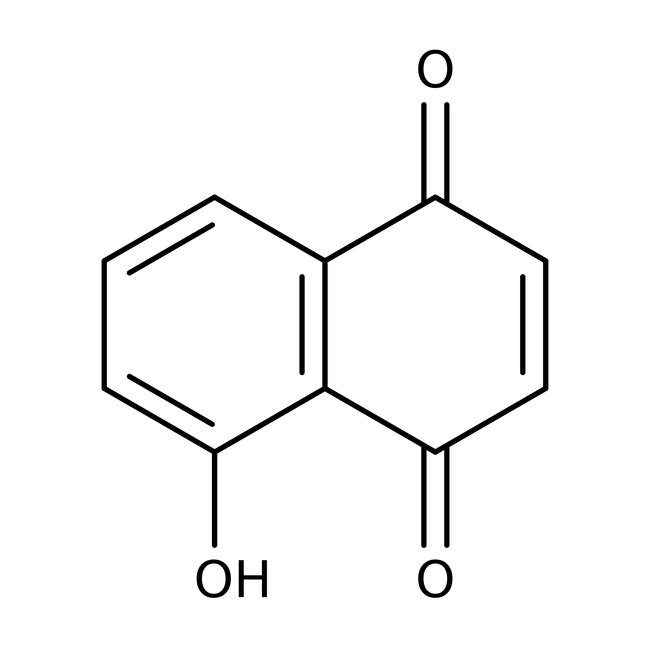
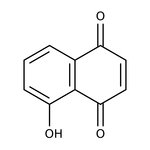
5-Hydroxy-1,4-naphthoquinone, 99%
CAS: 481-39-0 | C10H6O3 | 174.16 g/mol
Catalog number ALFH28343.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or Material5-Hydroxy-1,4-naphthoquinone
CAS481-39-0
Health Hazard 1H301-H315-H319-H335
Health Hazard 2GHS H Statement
H301-H315-H319-H335
Toxic if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H301-H315-H319-H335
Toxic if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P310-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
5-Hydroxy-1,4-naphthoquinone is used as a natural dye in cloth, fabrics, wool and ink. It is a coloring agent for food and cosmetics. It is involved in the synthesis of poly(hydroxyl-1,4-naphthoquinone) stabilized gold nanoparticles (AuNQ NPs), which is used for nonenzymatic electrochemical detection of glucose.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
5-Hydroxy-1,4-naphthoquinone is used as a natural dye in cloth, fabrics, wool and ink. It is a coloring agent for food and cosmetics. It is involved in the synthesis of poly(hydroxyl-1,4-naphthoquinone) stabilized gold nanoparticles (AuNQ NPs), which is used for nonenzymatic electrochemical detection of glucose.
Solubility
Soluble in alcohol, chloroform, dimethylsulfoxide, ether and aqueous solution of alkalies. Slightly soluble in water.
Notes
Light sensitive. Incompatible with strong bases, strong reducing agents and strong oxidizing agents.
5-Hydroxy-1,4-naphthoquinone is used as a natural dye in cloth, fabrics, wool and ink. It is a coloring agent for food and cosmetics. It is involved in the synthesis of poly(hydroxyl-1,4-naphthoquinone) stabilized gold nanoparticles (AuNQ NPs), which is used for nonenzymatic electrochemical detection of glucose.
Solubility
Soluble in alcohol, chloroform, dimethylsulfoxide, ether and aqueous solution of alkalies. Slightly soluble in water.
Notes
Light sensitive. Incompatible with strong bases, strong reducing agents and strong oxidizing agents.
RUO – Research Use Only
General References:
- Li, D.; Cheng, L.; Jin, B. Investigation on PCET-accompanied Dimerization of 5-hydroxy-1, 4-naphthoquinone in the Process of Electrochemical Reduction by In Situ FT-IR Spectroelectrochemistry and Density Functional Calculation. Electrochim. Acta 2014, 130, 387-396.
- Peng, X.; Nie, Y.; Wu, J.; Huang, Q.; Cheng, Y. Juglone prevents metabolic endotoxemia-induced hepatitis and neuroinflammation via suppressing TLR4F-κB signaling pathway in high-fat diet rats. Biochem. Biophys. Res. Commun. 2015, 462 (3), 245-250.