Search Thermo Fisher Scientific
Thermo Scientific Chemicals
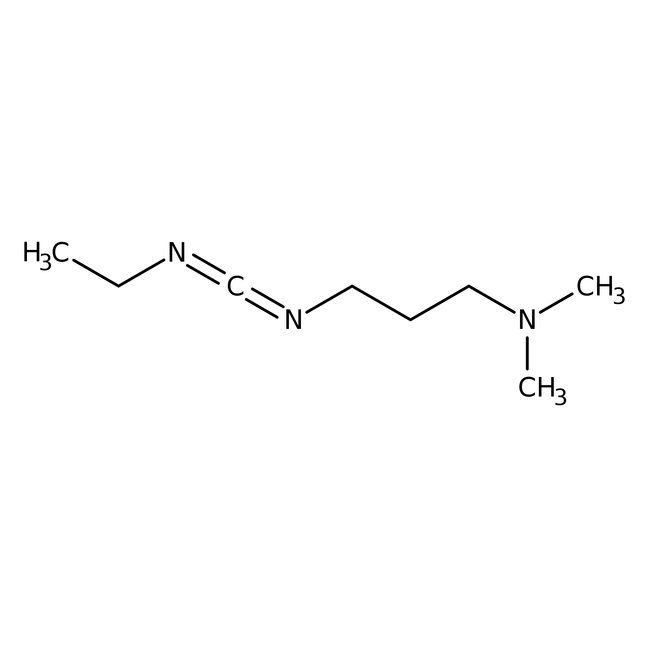
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide, 97%
CAS: 1892-57-5 | C8H17N3 | 155.245 g/mol
Catalog number ALFB25057.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide
CAS1892-57-5
Health Hazard 1H314-H335
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P260-P264b-P271-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P501c
View more
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide is used as a carboxyl activating agent and activate phosphate groups in phospho mono and di esters. It is used in peptide synthesis, 3'-amino-3'-deoxyadenosine-5'-di- and triphosphates and in the preparation of antibodies like immunoconjugates. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide is used as a carboxyl activating agent and activate phosphate groups in phospho mono and di esters. It is used in peptide synthesis, 3′-amino-3′-deoxyadenosine-5′-di- and triphosphates and in the preparation of antibodies like immunoconjugates. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
Solubility
Soluble in water.
Notes
Air sensitive. Incompatible with strong acids and strong oxidizing agents.
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide is used as a carboxyl activating agent and activate phosphate groups in phospho mono and di esters. It is used in peptide synthesis, 3′-amino-3′-deoxyadenosine-5′-di- and triphosphates and in the preparation of antibodies like immunoconjugates. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
Solubility
Soluble in water.
Notes
Air sensitive. Incompatible with strong acids and strong oxidizing agents.
RUO – Research Use Only
General References:
- Free base form of 'Water-soluble' carbodiimide. For applications see following entry. In the presence of CuCl2, (E)- or (Z)-2-substituted 3-hydroxy-3-phenylpropionate esters can be dehydrated stereoselectively to the corresponding (E) or (Z)-cinnamates: Tetrahedron Lett., 40, 5019 (1999).
- Li, J.; Yue, L.; Li, C.; Pan, Y.; Yang, L. Enantioselectivity and catalysis improvements of Pseudomonas cepacia lipase with Tyr and Asp modification. Catal. Sci. Technol. 2015, 5, 2681-2687.
- Lopez, R. J.; Babanova, S.; Artyushkova, K.; Atanassov, P. Surface modifications for enhanced enzyme immobilization and improved electron transfer of PQQ-dependent glucose dehydrogenase anodes. Bioelectrochemistry 2015, 105, 78-87.