Search Thermo Fisher Scientific
Thermo Scientific Chemicals
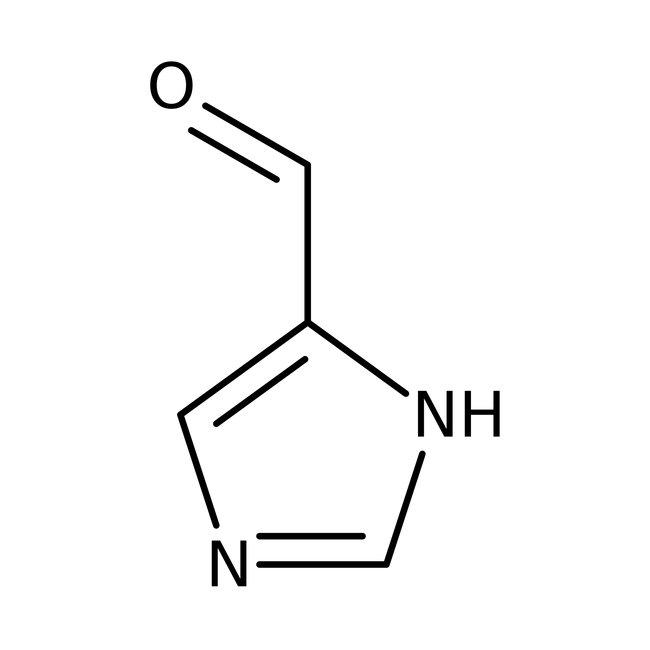
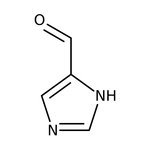
Imidazole-4-carboxaldehyde, 97%
CAS: 3034-50-2 | C4H4N2O | 96.089 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB25050.03 | 1 g |
Catalog number ALFB25050.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Specifications
Chemical Name or MaterialImidazole-4-carboxaldehyde
CAS3034-50-2
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
Imidazole-4-carboxaldehyde is a 4-formyl derivative of imidazole used in the preparation of C17,20-lyase inhibitor for the treatment of androgen-dependent prostate cancer. It is also used in the synthesis of other biologically active compounds such as antimalarial drugs, fabrication of colorimetric chemosensor.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Imidazole-4-carboxaldehyde is a 4-formyl derivative of imidazole used in the preparation of C17,20-lyase inhibitor for the treatment of androgen-dependent prostate cancer. It is also used in the synthesis of other biologically active compounds such as antimalarial drugs, fabrication of colorimetric chemosensor.
Solubility
Soluble in DMSO, methanol.
Notes
Air sensitive. Store under inert gas. Store away from oxidizing agents, air.
Imidazole-4-carboxaldehyde is a 4-formyl derivative of imidazole used in the preparation of C17,20-lyase inhibitor for the treatment of androgen-dependent prostate cancer. It is also used in the synthesis of other biologically active compounds such as antimalarial drugs, fabrication of colorimetric chemosensor.
Solubility
Soluble in DMSO, methanol.
Notes
Air sensitive. Store under inert gas. Store away from oxidizing agents, air.
RUO – Research Use Only
General References:
- Seon-Yeong Gwon; Sung-Hoon Kim. Anion sensing and F--induced reversible photoreaction of D-π-A type dye containing imidazole moiety as donor. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014, 117, 810-813.
- K Honda; Y Nishijima. Convenient synthesis of alkyl esters of urocanic acid. Journal of Pharmaceutical Sciences. 1981, 70 (1), 98-99.