Search Thermo Fisher Scientific
Thermo Scientific Chemicals
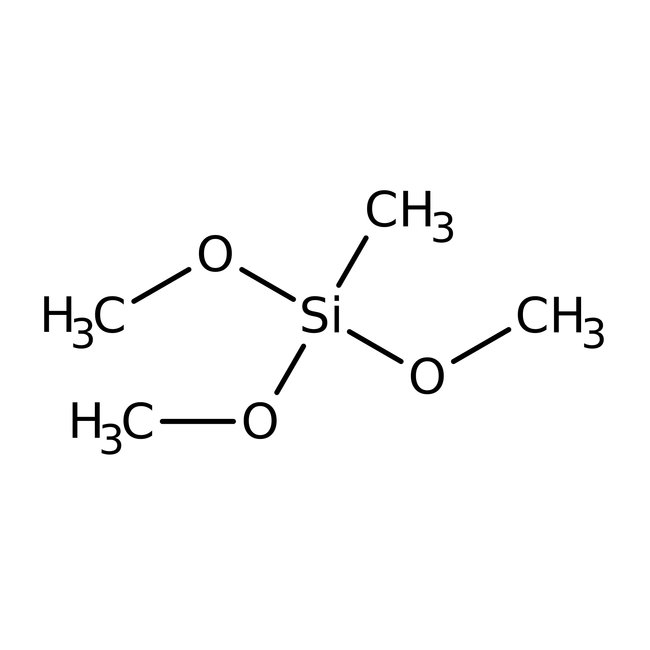
Methyltrimethoxysilane, 97%
CAS: 1185-55-3 | C4H12O3Si | 136.222 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB23594.AK | 250 mL |
Catalog number ALFB23594.AK
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 mL
Specifications
Boiling Point102°C to 103°C
Chemical Name or MaterialMethyltrimethoxysilane
CAS1185-55-3
Health Hazard 1H225-H315-H319-H335
Health Hazard 2GHS H Statement
H225-H315-H319-H335
Highly flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H225-H315-H319-H335
Highly flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
Methyltrimethoxysilane is used as an acid scavenger, for example in the formation of substituted azulenes from allenylsilanes and tropylium tetrafluoroborate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methyltrimethoxysilane is used as an acid scavenger, for example in the formation of substituted azulenes from allenylsilanes and tropylium tetrafluoroborate.
Solubility
Decomposes in water.
Notes
Moisture Sensitive. Store under dry inert gas. Store away from moisture, strong bases and oxidizing agents. Protect from humidity and water.
Methyltrimethoxysilane is used as an acid scavenger, for example in the formation of substituted azulenes from allenylsilanes and tropylium tetrafluoroborate.
Solubility
Decomposes in water.
Notes
Moisture Sensitive. Store under dry inert gas. Store away from moisture, strong bases and oxidizing agents. Protect from humidity and water.
RUO – Research Use Only
General References:
- Eiichi Tabei; Shigeru Mori; Fumio Okada; Susumu Tajima; Kazuo Ogino; Yuzuru Okawara and Seiji Tobita. Metastable ion study of organosilicon compounds. Part V—tetramethoxysilane and trimethoxymethylsilane. Organic Mass Spectrometry. 1992, 27, 702-708.
- Has been used as an acid scavenger, for example in the formation of substituted azulenes from allenylsilanes and Tropyl ium tetrafluoroborate, A14045: J. Am. Chem. Soc., 111, 389 (1989).