Search Thermo Fisher Scientific
Thermo Scientific Chemicals
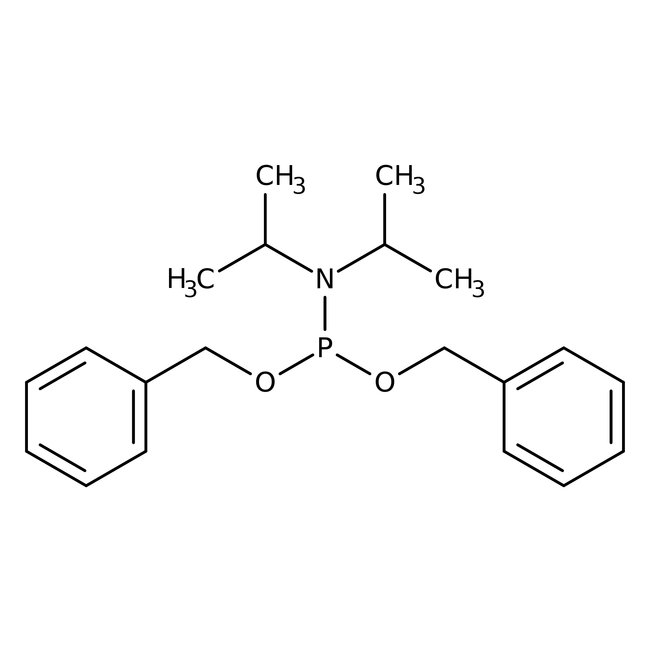
Dibenzyl diisopropylphosphoramidite, 90+%
CAS: 108549-23-1 | C20H28NO2P | 345.423 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB22629.06 | 5 g |
Catalog number ALFB22629.06
Price (MYR)
804.00
EA
Quantity:
5 g
Price (MYR)
804.00
EA
Specifications
Chemical Name or MaterialDibenzyl diisopropylphosphoramidite
CAS108549-23-1
Health Hazard 1H227-H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P235-P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P370+P378q-P501c
View more
Dibenzyl N,N-diisopropylphosphoramidite may be used for the preparation of phosphopeptides. It is used for the synthesis of a GDP (guanosine diphosphate) analog, SML-8-73-1. It is useful for nucleotide coupling.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Dibenzyl N,N-diisopropylphosphoramidite may be used for the preparation of phosphopeptides. It is used for the synthesis of a GDP (guanosine diphosphate) analog, SML-8-73-1. It is useful for nucleotide coupling.
Solubility
Soluble in chloroform.
Notes
Moisture sensitive. Store under inert gas. Store at 0°C. Incompatible with oxidizing agents, water/ moisture.
Dibenzyl N,N-diisopropylphosphoramidite may be used for the preparation of phosphopeptides. It is used for the synthesis of a GDP (guanosine diphosphate) analog, SML-8-73-1. It is useful for nucleotide coupling.
Solubility
Soluble in chloroform.
Notes
Moisture sensitive. Store under inert gas. Store at 0°C. Incompatible with oxidizing agents, water/ moisture.
RUO – Research Use Only
General References:
- D L Clemm; L Sherman; V Boonyaratanakornkit; W T Schrader; N L Weigel; D P Edwards. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Molecular Endocrinology. 2000, 14 (1), 52-65.
- Sang Min Lim; Kenneth D Westover; Scott B Ficarro; Rane A Harrison; Hwan Geun Choi; Michael E Pacold; Martin Carrasco; John Hunter; Nam Doo Kim; Ting Xie; Taebo Sim, Pasi A Jänne; Matthew Meyerson; Jarrod A Marto; John R Engen; Nathanael S Gray. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angewandte Chemie. 2014, 53 (1), 199-204.
- Phosphitylating agent which reacts with an alcohol under mild conditions. Subsequent oxidation, e.g. with hydrogen peroxide, followed by hydrogenolysis of the benzyl groups, affords the alkyl phosphate: Tetrahedron Lett., 29, 979 (1988).