Search Thermo Fisher Scientific
Thermo Scientific Chemicals
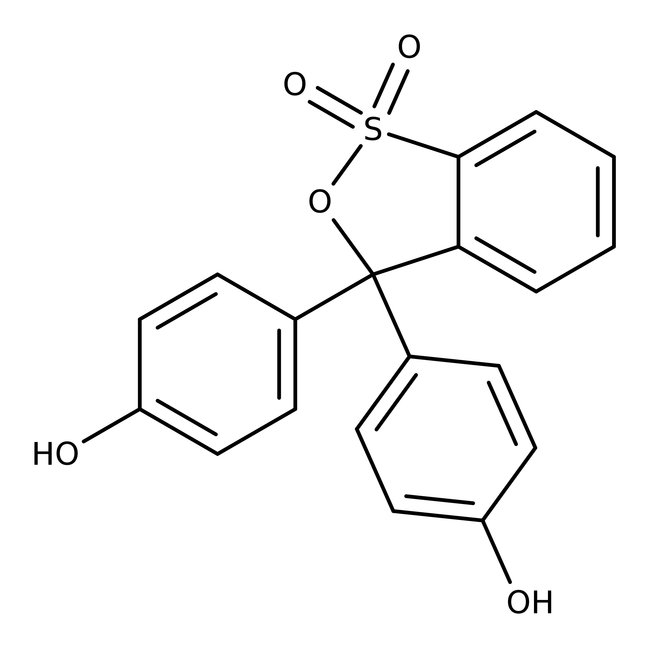
Phenol Red
CAS: 143-74-8 | C19H14O5S | 354.38 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB21710.09 | 10 g |
Catalog number ALFB21710.09
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
10 g
Specifications
Chemical Name or MaterialPhenol Red
CAS143-74-8
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
Phenol red is used as a non absorbed marker in gravimetric method. It is a pH indicator used in cell biology laboratories and in home swimming pool test kits. It is used to check the kidney function based on the excreted phenol red by colorimetric method.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phenol red is used as a non absorbed marker in gravimetric method. It is a pH indicator used in cell biology laboratories and in home swimming pool test kits. It is used to check the kidney function based on the excreted phenol red by colorimetric method.
Solubility
Soluble in water, ethanol and methanol. Insoluble in chloroform.
Notes
Incompatible with strong oxidizing agents.
Phenol red is used as a non absorbed marker in gravimetric method. It is a pH indicator used in cell biology laboratories and in home swimming pool test kits. It is used to check the kidney function based on the excreted phenol red by colorimetric method.
Solubility
Soluble in water, ethanol and methanol. Insoluble in chloroform.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Acid-base indicator: approx pH 6.8 - 8.2.
- Chauhan, S. S.; Jasra, R. V.; Sharma, A. L. Phenol red dye functionalized nanostructured silica films as optical filters and pH sensors. Ind. Eng. Chem. Res. 2012, 51 (31), 10381-10389.
- Ghaedi, M.; Daneshfar, A.; Ahmadi, A.; Momeni, M. S. Artificial neural network-genetic algorithm based optimization for the adsorption of phenol red (PR) onto gold and titanium dioxide nanoparticles loaded on activated carbon. J. Ind. Eng. Chem. 2015, 21, 587-598.