Search Thermo Fisher Scientific
Thermo Scientific Chemicals
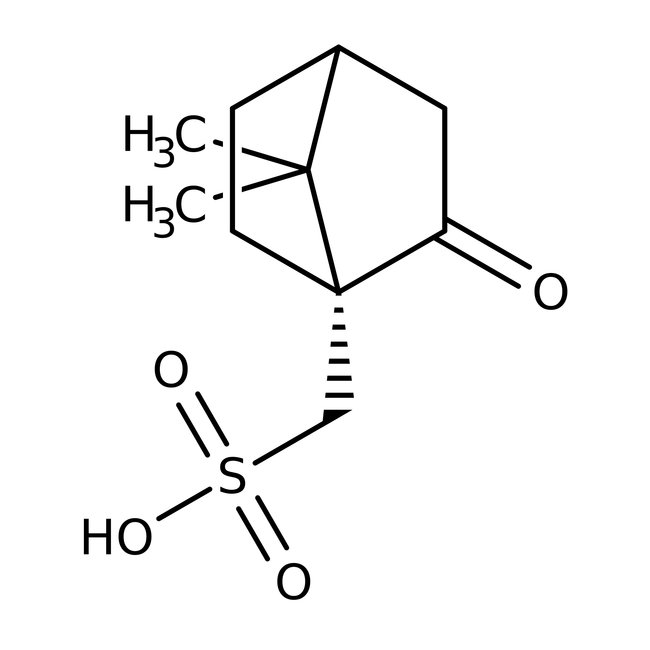
(1R)-(-)-Camphor-10-sulfonic acid, 98%
CAS: 35963-20-3 | C10H16O4S | 232.29 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB21553.22 | 100 g |
Catalog number ALFB21553.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material(1R)-(-)-Camphor-10-sulfonic acid
CAS35963-20-3
Health Hazard 1H314
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P260-P264b-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P501c
View more
(1R)-(-)-Camphor-10-sulfonic acid, is used as a pharamaceutical intermediate and also used as a chiral derivative of Camp. It can also be used as resolving agents for chiral amines and other cations.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(1R)-(-)-Camphor-10-sulfonic acid, is used as a pharamaceutical intermediate and also used as a chiral derivative of Camp. It can also be used as resolving agents for chiral amines and other cations.
Solubility
Soluble in water, and a wide variety of organic substances.
Notes
Hygroscopic. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Keep away strong oxidizing agents, strong acids and bases.
(1R)-(-)-Camphor-10-sulfonic acid, is used as a pharamaceutical intermediate and also used as a chiral derivative of Camp. It can also be used as resolving agents for chiral amines and other cations.
Solubility
Soluble in water, and a wide variety of organic substances.
Notes
Hygroscopic. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Keep away strong oxidizing agents, strong acids and bases.
RUO – Research Use Only
General References:
- W Oppolzer.; P Dudfield. Asymmetric halogenation of camphor-10-sulfonic acid derived esters: an efficient new route to enantiomerically pure halohydrins and epoxides. Tetrahedron letters. 198526 (41), 5036-5040.
- M Vandewalle.; J Van der Eycken.; W Oppolzer. Iridoids: enantioselective synthesis of loganin via an asymmetric diels-alder reaction. Tetrahedron. 198642 (14), 4035-4043.