Search Thermo Fisher Scientific
Thermo Scientific Chemicals
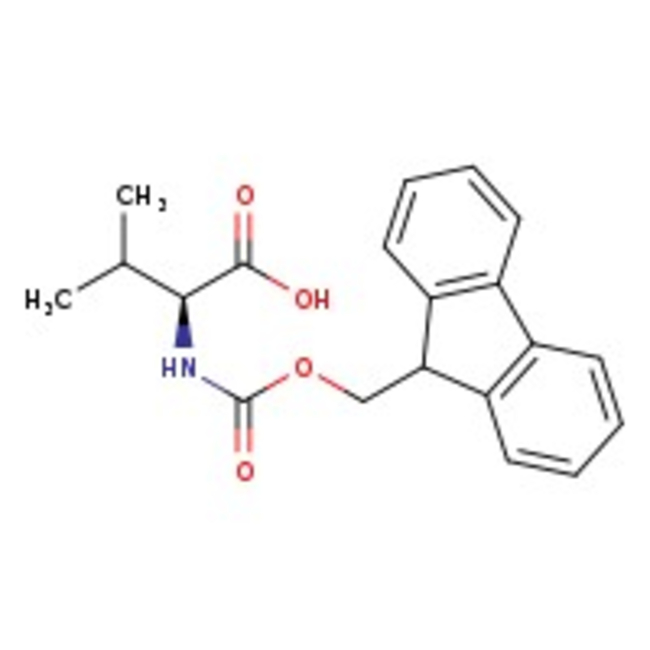
N-Fmoc-L-valine, 98%
CAS: 68858-20-8 | C20H21NO4 | 339.39 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB21030.14 | 25 g |
Catalog number ALFB21030.14
Price (MYR)
522.00
EA
Quantity:
25 g
Price (MYR)
522.00
EA
Specifications
Chemical Name or MaterialN-Fmoc-L-valine
CAS68858-20-8
Health Hazard 1H315-H319-H335
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
Melting Point142°C to 147°C
View more
It is potentially useful for proteomics studies and solid phase peptide synthesis techniques. Fmoc-valine (in addition to the other amino acids) is commonly used to synthesize 4-thiazolidinones (e.g. (E)-5-(4-Ethylbenzylidene)-2-thioxothiazolidin-4-one [E925745]) and 4-metathiazanones as well.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is potentially useful for proteomics studies and solid phase peptide synthesis techniques. Fmoc-valine (in addition to the other amino acids) is commonly used to synthesize 4-thiazolidinones (e.g. (E)-5-(4-Ethylbenzylidene)-2-thioxothiazolidin-4-one [E925745]) and 4-metathiazanones as well.
Solubility
Solubility in methanol gives very faint turbidity.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
It is potentially useful for proteomics studies and solid phase peptide synthesis techniques. Fmoc-valine (in addition to the other amino acids) is commonly used to synthesize 4-thiazolidinones (e.g. (E)-5-(4-Ethylbenzylidene)-2-thioxothiazolidin-4-one [E925745]) and 4-metathiazanones as well.
Solubility
Solubility in methanol gives very faint turbidity.
Notes
Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Albert Bowers, et al. Total Synthesis and Biological Mode of Action of Largazole: A Potent Class I Histone Deacetylase Inhibitor.J. Am. Chem. Soc.,2008130(33),11219-11222.
- Shu-Li You , et al. Highly Efficient Biomimetic Total Synthesis and Structural Verification of Bistratamides E and J from Lissoclinum bistratum.Chem. Eur. J.,2004,10(1), 71-75.