Search Thermo Fisher Scientific
Thermo Scientific Chemicals
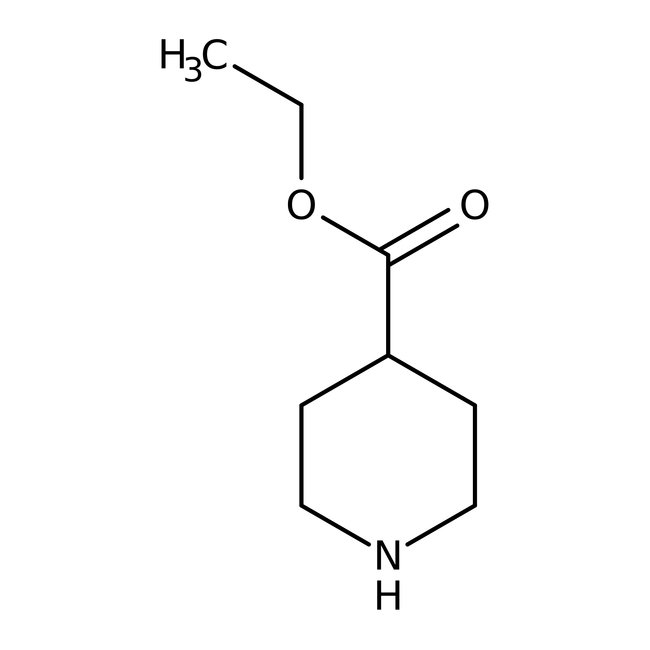
Ethyl isonipecotate, 98%
CAS: 1126-09-6 | C8H15NO2 | 157.21 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB20601.22 | 100 g |
Catalog number ALFB20601.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialEthyl isonipecotate
CAS1126-09-6
Health Hazard 1H227-H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P235-P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P370+P378q-P501c
View more
It is used as a reactant for synthesis of SMN protein modulators, β-aryl and β-amino-substituted aliphatic esters by rhodium catalyzed tandem double bond migration/conjugate addition, nitroethylenediamines by nucleophilic ring opening of nitroimidazolidinone. It is also involved in the reactions of RhoA inhibitors for cardiovascular disease therapy and saccharin derived Mannich bases as antimicrobials and antioxidants. It is employed as a reactant for one-pot reductive amination and Suzuki-Miyaura cross coupling of formyl aryl and heteroaryl MIDA boronates.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Solubility
Fully miscible with water.
Notes
Store at room temperature. Incompatible with strong oxidizing agents.
Fully miscible with water.
Notes
Store at room temperature. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Samantha J. Barry.; Richard M. Carr.; Stephen J. Lane.; William J. Leavens.; Soraya Monté.; Ian Waterhouse. Derivatisation for liquid chromatography/electrospray mass spectrometry: synthesis of pyridinium compounds and their amine and carboxylic acid derivatives.rapid communications in mass spectroscopy. 2003 , 17 (6),603-620 .
- Stephen A. Kolodziej.; Susan L. Hockerman.; Gary A. DeCrescenzo.; Joseph J. McDonald.; Debbie A. Mischke.; Grace E. Munie.; Theresa R. Fletcher.; Nathan Stehle.; Craig Swearingen.; Daniel P. Becker. MMP-13 selective isonipecotamide α-sulfone hydroxamates.Bioorg. Med. Chem. Lett. 2010, 20 (12),3561-3564 .