Search Thermo Fisher Scientific
Thermo Scientific Chemicals
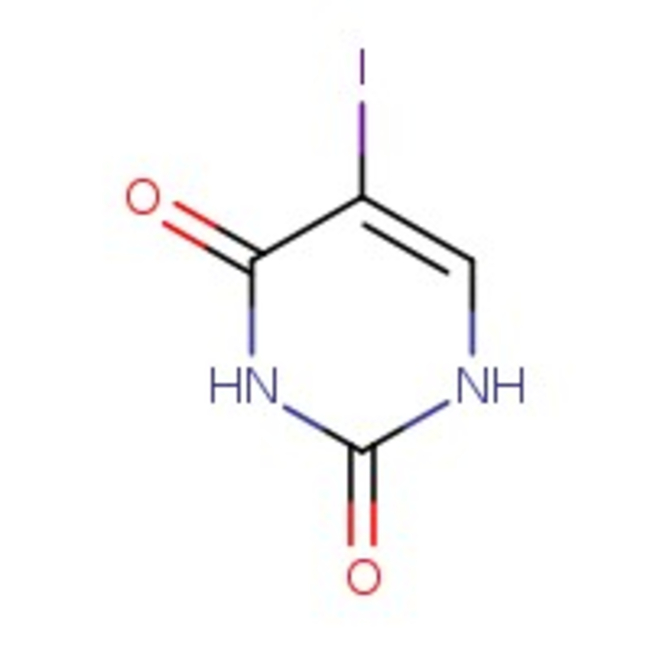
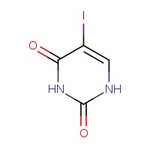
5-Iodouracil, 97%
CAS: 696-07-1 | C4H3IN2O2 | 237.98 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA18994.18 | 50 g |
Catalog number ALFA18994.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Specifications
Chemical Name or Material5-Iodouracil
CAS696-07-1
Health Hazard 1H302+H312+H332-H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
It is used as an intermediate. It has an antitumor activity.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used as an intermediate. It has an antitumor activity.
Solubility
Very faint turbidity in NH3aq. Soluble in 1M NaOH.
Notes
Light Sensitive. Store away from oxidizing agents and light. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
It is used as an intermediate. It has an antitumor activity.
Solubility
Very faint turbidity in NH3aq. Soluble in 1M NaOH.
Notes
Light Sensitive. Store away from oxidizing agents and light. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Morris J. Robins.; Philip J. Barr.Nucleic acid related compounds. 31. Smooth and efficient palladium-copper catalyzed coupling of terminal alkynes with 5-iodouracil nucleosides.Tetrahedron Lett.198122(5), 421-424.
- MC Willis.; BJ Hicke.; OC Uhlenbeck.; TR Cech.; TH Koch. Photocrosslinking of 5-iodouracil-substituted RNA and DNA to proteins.Science.1993,262(5137), 1255-1257.
- For a review of the use of uracils as starting materials in heterocyclic synthesis, see: Adv. Het. Chem., 55, 130 (1992).