Search Thermo Fisher Scientific
Thermo Scientific Chemicals
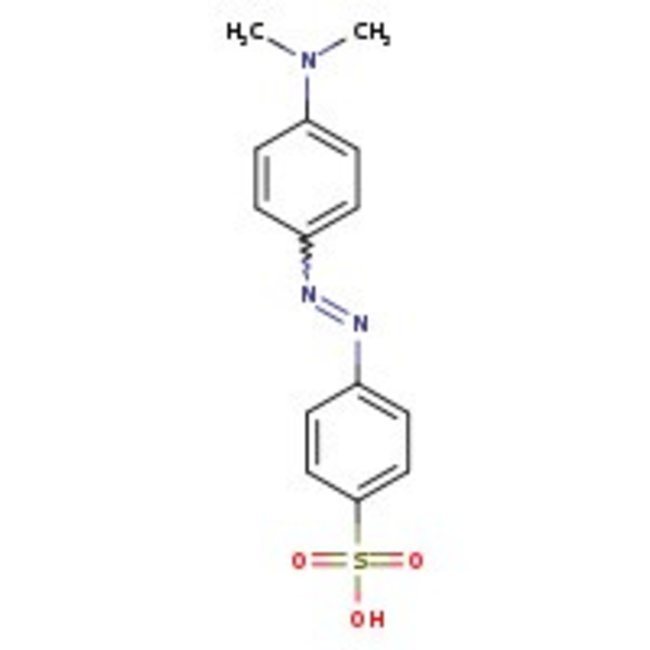
Methyl Orange
CAS: 547-58-0 | C14H14N3NaO3S | 327.334 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA17604.22 | 100 g |
Catalog number ALFA17604.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialMethyl Orange
Melting Point>300°C
CAS547-58-0
Health Hazard 1Danger
Health Hazard 2GHS H Statement
H301
Toxic if swallowed.
H301
Toxic if swallowed.
View more
Methyl Orange is a pH indicator used in titrations for acids. It is also used for histological microscopy. Further, it is used as counter stain to crystal violet in staining pollen tubes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methyl Orange is a pH indicator used in titrations for acids. It is also used for histological microscopy. Further, it is used as counter stain to crystal violet in staining pollen tubes.
Solubility
Soluble in ethanol. Partially soluble in hot water. Slightly soluble in cold water and pyrimidine. Insoluble in ether and alcohol.
Notes
Incompatible with strong oxidizing agents.
Methyl Orange is a pH indicator used in titrations for acids. It is also used for histological microscopy. Further, it is used as counter stain to crystal violet in staining pollen tubes.
Solubility
Soluble in ethanol. Partially soluble in hot water. Slightly soluble in cold water and pyrimidine. Insoluble in ether and alcohol.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Acid-base indicator: pH 3.2 - 4.4.
- Gomez-Solís, C.; Ballesteros, J. C.; Torres-Martínez, L. M.; Juárez-Ramírez, I.; Torres, L. A. D.; Zarazua-Morin, M. E.; Lee, S. W. Rapid synthesis of ZnO nano-corncobs from Nital solution and its application in the photodegradation of methyl orange. J. Photochem. Photobiol., A 2015, 298, 49-54.
- Varga, M.; Kopecká, J.; Morávková, Z.; Křivka, I.; Trchová, M.; Stejskal, J.; Prokeš, J. Effect of oxidant on electronic transport in polypyrrole nanotubes synthesized in the presence of methyl orange. J. Polym. Sci., Part B: Polym. Phys. 2015, 53 (16), 1147-1159.