Search Thermo Fisher Scientific
Thermo Scientific Chemicals
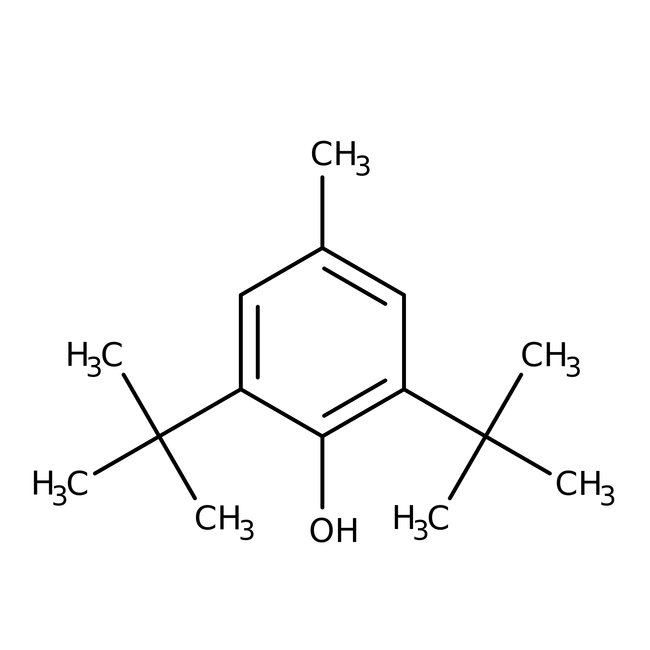
2,6-Di-tert-butyl-4-methylphenol, 99%
CAS: 128-37-0 | C15H24O | 220.356 g/mol
Catalog number ALFA16863.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or Material2,6-Di-tert-butyl-4-methylphenol
CAS128-37-0
Health Hazard 1H335-H373
Health Hazard 2GHS H Statement
H302-H319
Harmful if swallowed.
Causes serious eye irritation.
H302-H319
Harmful if swallowed.
Causes serious eye irritation.
Health Hazard 3P260-P271-P304+P340-P312-P314-P501c
View more
2,6-Di-tert-butyl-4-methylphenol is used as an antioxidant for cosmetics, vitamins, pharmaceuticals, rubber, oils and fats. It is used as a fuel additive in the petroleum industry and also used in hydraulic fluids, turbine and gear oils and jet fuels. It acts as a stabilizer in diethyl ether, tetrahydrofuran and other laboratory chemicals to prevent peroxide formation. It is effectively involved as a polymerization inhibitor in the process of oxidation of allyl alcohol to glycerine. It is utilized in the synthesis of organoaluminum compound, methylaluminum bis(2,6-di-tert-butyl-4-alkylphenoxide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,6-Di-tert-butyl-4-methylphenol is used as an antioxidant for cosmetics, vitamins, pharmaceuticals, rubber, oils and fats. It is used as a fuel additive in the petroleum industry and also used in hydraulic fluids, turbine and gear oils and jet fuels. It acts as a stabilizer in diethyl ether, tetrahydrofuran and other laboratory chemicals to prevent peroxide formation. It is effectively involved as a polymerization inhibitor in the process of oxidation of allyl alcohol to glycerine. It is utilized in the synthesis of organoaluminum compound, methylaluminum bis(2,6-di-tert-butyl-4-alkylphenoxide.
Solubility
Soluble in methanol, toluene, isopropanol, methyl ethyl ketone, acetone, benzene, petroleum ether and ethanol. Insoluble in water.
Notes
Light-sensitive. Keep the container tightly closed in a cool, dry and well-ventilated place. Incompatible with acid chlorides, acid anhydrides, oxidizing agents, bases, brass, copper and copper alloys.
2,6-Di-tert-butyl-4-methylphenol is used as an antioxidant for cosmetics, vitamins, pharmaceuticals, rubber, oils and fats. It is used as a fuel additive in the petroleum industry and also used in hydraulic fluids, turbine and gear oils and jet fuels. It acts as a stabilizer in diethyl ether, tetrahydrofuran and other laboratory chemicals to prevent peroxide formation. It is effectively involved as a polymerization inhibitor in the process of oxidation of allyl alcohol to glycerine. It is utilized in the synthesis of organoaluminum compound, methylaluminum bis(2,6-di-tert-butyl-4-alkylphenoxide.
Solubility
Soluble in methanol, toluene, isopropanol, methyl ethyl ketone, acetone, benzene, petroleum ether and ethanol. Insoluble in water.
Notes
Light-sensitive. Keep the container tightly closed in a cool, dry and well-ventilated place. Incompatible with acid chlorides, acid anhydrides, oxidizing agents, bases, brass, copper and copper alloys.
RUO – Research Use Only
General References:
- Radical inhibitor and antioxidant, readily soluble in nonpolar media. Finds widespread use in the inhibition of peroxide formation in ether solvents and also as a preservative/ antioxidant for unsaturated oils and fats in the food, synthetic rubber and paint industries.
- Source of t-butyl group in aromatic substitution reactions. For reviews of the use of t-butyl as a positional protecting group for aromatic substitution reactions, readily removable with, e.g. AlCl 3 in toluene, see: Org. Prep. Proced. Int., 8, 51 (1976); Synthesis, 921 (1979).
- Khabibullina, G. A.; Belyaeva, A. S.; Niyazov, N. A.; Movsum-zade, E. M. Simple and low-cost method for processing of waste from production of 2,6-di-tert -butyl-4-methylphenol to give a high-efficiency stabilizer of rubbers and biodiesel fuel. Russ. J. Appl. Chem. 2014, 87 (11), 1680-1685.
- Prishchenko, A. A.; Livantsov, M. V.; Novikova, O. P.; Livantsova, L. I.; Milaeva, E. R. Synthesis of Organophosphorus-Substituted Amides of Carbonic Acids with PCHNC(O) and 2,6-Di-tert-butyl-4-methylphenol Fragments. Heteroat. Chem. 2008, 19 (7), 733-737.