Search Thermo Fisher Scientific
Thermo Scientific Chemicals
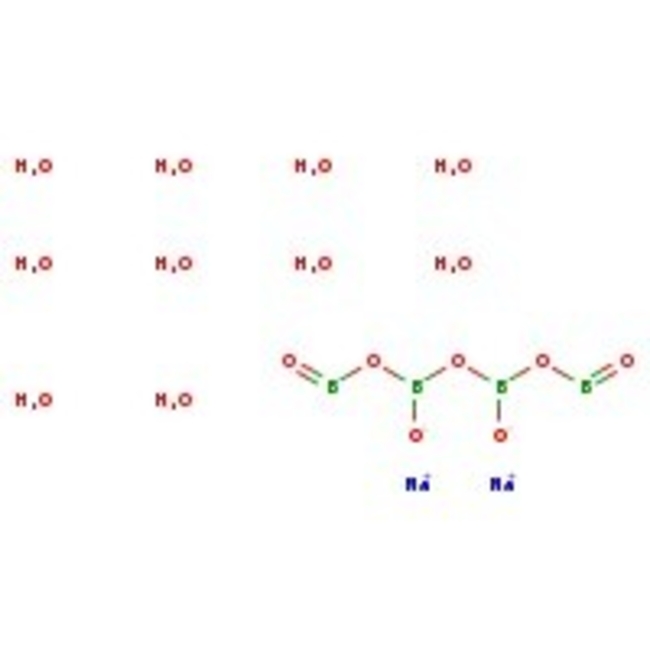
Sodium tetraborate decahydrate, 99+%
CAS: 1303-96-4 | B4Na2O7·10H2O
Catalog number ALFA16176.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialSodium tetraborate decahydrate
Melting Point75°C
CAS1303-96-4
Health Hazard 1H319-H360FD
Health Hazard 2GHS H Statement
H360
May damage fertility or the unborn child.
H360
May damage fertility or the unborn child.
View more
It is used in detergents, cosmetics, and enamel glazes. It is likewise employed to prepare buffer solutions in biochemistry, as a fire retardant, anti-fungal compound, in the manufacture of fiberglass, as a flux in metallurgy, neutron-capture shields for radioactive sources, a texturing agent in cooking, and as a harbinger for other boron compounds. The molten borax bath is useful for carbide coating process on metals. It is applied as an efficient metal-free catalyst for Hetero-Michael reactions in an aqueous medium.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used in detergents, cosmetics, and enamel glazes. It is likewise employed to prepare buffer solutions in biochemistry, as a fire retardant, anti-fungal compound, in the manufacture of fiberglass, as a flux in metallurgy, neutron-capture shields for radioactive sources, a texturing agent in cooking, and as a harbinger for other boron compounds. The molten borax bath is useful for carbide coating process on metals. It is applied as an efficient metal-free catalyst for Hetero-Michael reactions in an aqueous medium.
Solubility
Soluble in water, glycerol and ethanol. Insoluble in acids
Notes
Incompatible with strong oxidizing agents and strong acids.
It is used in detergents, cosmetics, and enamel glazes. It is likewise employed to prepare buffer solutions in biochemistry, as a fire retardant, anti-fungal compound, in the manufacture of fiberglass, as a flux in metallurgy, neutron-capture shields for radioactive sources, a texturing agent in cooking, and as a harbinger for other boron compounds. The molten borax bath is useful for carbide coating process on metals. It is applied as an efficient metal-free catalyst for Hetero-Michael reactions in an aqueous medium.
Solubility
Soluble in water, glycerol and ethanol. Insoluble in acids
Notes
Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Nasir, M. Z. M.; Sofer, Z.; Ambrosi, A.; Pumera, M. A limited anodic and cathodic potential window of MoS2: limitations in electrochemical applications. Nanoscale 2015, 7 (7), 3126-3129.
- Hussain, S.; Bharadwaj, S. K.; Chaudhuri, M. K.; Kalita, H. Borax as an Efficient Metal-Free Catalyst for Hetero-Michael Reactions in an Aqueous Medium. Eur. J. org. Chem. 2007, 2007 (2), 374-378.