Search Thermo Fisher Scientific
Thermo Scientific Chemicals
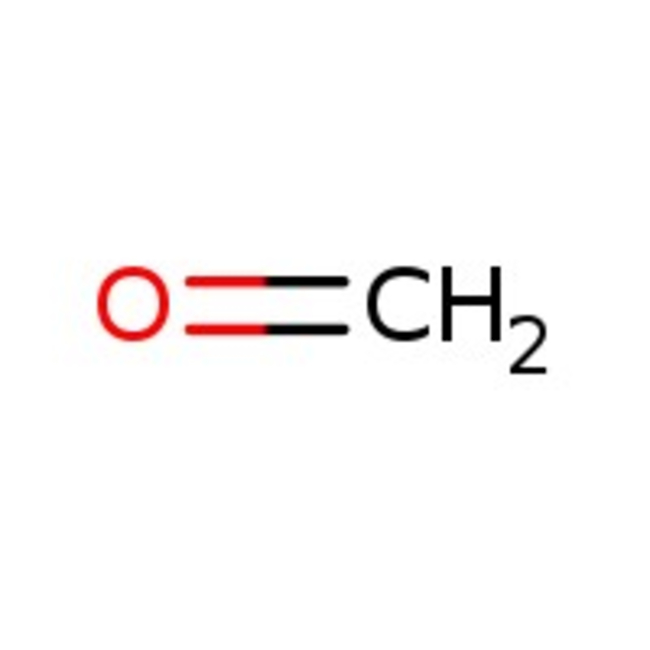
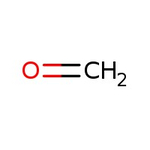
Formaldehyde, 37% w/w aq. soln., stab. with 7-8% methanol
CAS: 50-00-0 | CH2O | 30.03 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA16163.AP | 500 mL |
Catalog number ALFA16163.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or MaterialFormaldehyde
Name Notestabilized with 7 to 8% methanol
CAS50-00-0
Health Hazard 1H226-H301+H311+H331-H314-H317-H335-H336-H341-H350-H370-H372
Health Hazard 2GHS H Statement
H301-H311-H331-H314-H226-H351-H371-H317
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Causes severe skin burns and eye damage.
Flammable liquid and vapour.
Suspected of causing cancer.
May cause damage to organs.
May cause an allergic skin reaction.
H301-H311-H331-H314-H226-H351-H371-H317
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Causes severe skin burns and eye damage.
Flammable liquid and vapour.
Suspected of causing cancer.
May cause damage to organs.
May cause an allergic skin reaction.
View more
Formaldehyde is used as a denaturant in formaldehyde-agarose gel electrophoresis of RNA. It is also used in fiber board, plywood, cigarette smoke, fuel burning appliances, kerosene space heaters and in photography. It acts as a precursor in the manufacturing of automobiles and used to make components for the transmission, electrical system, engine block, door panels, axles and brake shoes. It serves as a disinfectant. It also plays an ingredient in glues and as a preservative in medical laboratories as embalming fluid and as a sterilizer.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Formaldehyde is used as a denaturant in formaldehyde-agarose gel electrophoresis of RNA. It is also used in fiber board, plywood, cigarette smoke, fuel burning appliances, kerosene space heaters and in photography. It acts as a precursor in the manufacturing of automobiles and used to make components for the transmission, electrical system, engine block, door panels, axles and brake shoes. It serves as a disinfectant. It also plays an ingredient in glues and as a preservative in medical laboratories as embalming fluid and as a sterilizer.
Solubility
Soluble in water.
Notes
Incompatible with oxidizing agents.
Formaldehyde is used as a denaturant in formaldehyde-agarose gel electrophoresis of RNA. It is also used in fiber board, plywood, cigarette smoke, fuel burning appliances, kerosene space heaters and in photography. It acts as a precursor in the manufacturing of automobiles and used to make components for the transmission, electrical system, engine block, door panels, axles and brake shoes. It serves as a disinfectant. It also plays an ingredient in glues and as a preservative in medical laboratories as embalming fluid and as a sterilizer.
Solubility
Soluble in water.
Notes
Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Numerous examples of the use of aqueous formaldehyde are to be found in Organic Syntheses. See also Paraformaldehyde, A11313, and 1,3,5-Trioxane, A15639.
- Liu, X.; Guo, Z.; Roache, N. F.; Mocka, C. A.; Allen, M. R.; Mason, M. A. Henry’s Law Constant and Overall Mass Transfer Coefficient for Formaldehyde Emission from Small Water Pools under Simulated Indoor Environmental Conditions. Environ. Sci. Technol. 2015, 49(3), 1603-1610.
- Bahmanpour, A. M.; Hoadley, A.; Tanksale, A. Formaldehyde production via hydrogenation of carbon monoxide in the aqueous phase. Green Chem. 2015, 17 (6), 3500-3507.