Search Thermo Fisher Scientific
Thermo Scientific Chemicals
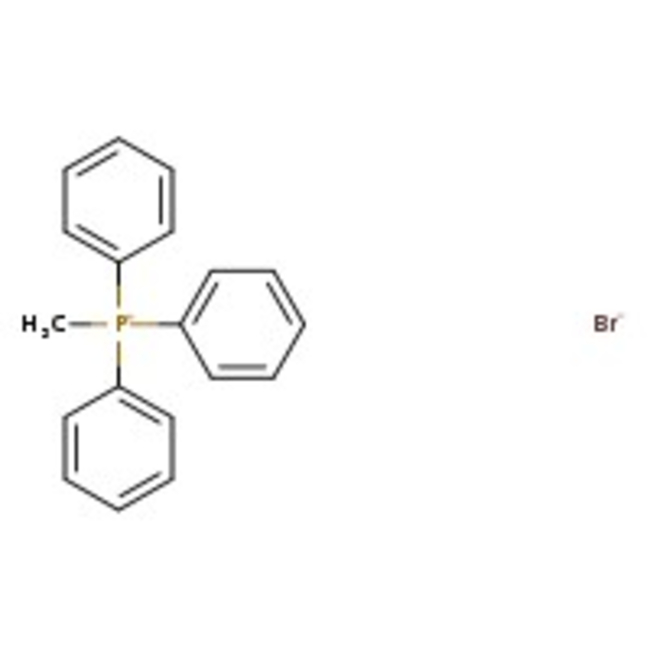
Methyltriphenylphosphonium bromide, 98+%
CAS: 1779-49-3 | C19H18BrP | 357.23 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15878.18 | 50 g |
Catalog number ALFA15878.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Specifications
Chemical Name or MaterialMethyltriphenylphosphonium bromide
CAS1779-49-3
Health Hazard 1H301-H335-H500
Health Hazard 2GHS H Statement
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P301+P310-P304+P340-P312-P330-P501c
View more
Methyltriphenylphosphonium bromide is used for methylenation through the Wittig reaction. It is utilized in the synthesis of an enyne and 9-isopropenyl -phenanthrene by using sodium amide as reagent. It is a lipophilic molecule and used to deliver molecules to specific cell components. Further, it acts as an antineoplastic agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methyltriphenylphosphonium bromide is used for methylenation through the Wittig reaction. It is utilized in the synthesis of an enyne and 9-isopropenyl -phenanthrene by using sodium amide as reagent. It is a lipophilic molecule and used to deliver molecules to specific cell components. Further, it acts as an antineoplastic agent.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.
Methyltriphenylphosphonium bromide is used for methylenation through the Wittig reaction. It is utilized in the synthesis of an enyne and 9-isopropenyl -phenanthrene by using sodium amide as reagent. It is a lipophilic molecule and used to deliver molecules to specific cell components. Further, it acts as an antineoplastic agent.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Precursor of Wittig ylide for methylenation of carbonyl compounds (see Appendix 1). See, e.g.: Org. Synth. Coll., 5, 751 (1973). A simplified procedure using K2CO3 in triglyme has been reported: Synth. Commun., 22, 513 (1992).
- Where the ylide is insufficiently reactive, a further proton can be removed by s-BuLi or t-BuLi, and the resulting lithio ylide reacted with hindered ketones such as fenchone: J. Am. Chem. Soc., 104, 4724 (1982). Reaction of the lithio ylide with an epoxide generates a new ylide, with which Wittig olefination of aldehydes gives trans-homoallylic alcohols (same ref.):
- Aissaoui, T.; AlNashef, I. M.; Hayyan, M.; Hashim, M. A. Neoteric FT-IR investigation on the functional groups of phosphonium-based deep eutectic solvents. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 149, 588-591.
- Chou, Y. H.; Takasugi, S.; Goseki, R.; Ishizone, T.; Chen, W. C. Nonvolatile organic field-effect transistor memory devices using polymer electrets with different thiophene chain lengths. Polym. Chem. 2014, 5 (3), 1063-1071.