Search Thermo Fisher Scientific
Thermo Scientific Chemicals
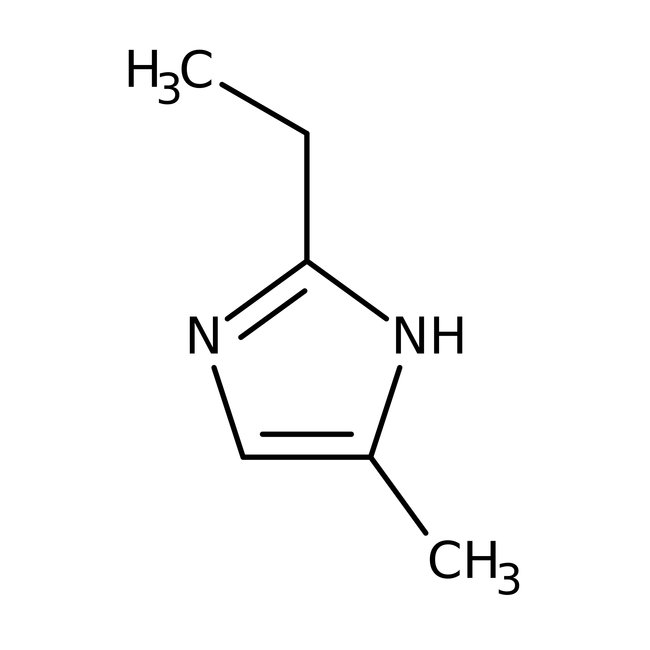
2-Ethyl-4-methylimidazole, 96%
CAS: 931-36-2 | C6H10N2 | 110.16 g/mol
Catalog number ALFA15798.22
Price (MYR)
1,150.00
EA
Quantity:
100 g
Price (MYR)
1,150.00
EA
Specifications
Chemical Name or Material2-Ethyl-4-methylimidazole
CAS931-36-2
Health Hazard 1H302-H315-H317-H318-H335-H351
Health Hazard 2GHS H Statement
H318-H302-H335-H315
Causes serious eye damage.
Harmful if swallowed.
May cause respiratory irritation.
Causes skin irritation.
H318-H302-H335-H315
Causes serious eye damage.
Harmful if swallowed.
May cause respiratory irritation.
Causes skin irritation.
Health Hazard 3P201-P202-P261-P264b-P270-P272-P280g-P281-P301+P312-P302+P352-P305+P351+P338-P308+P313-P310-P330-P333+P313-P362-P501c
View more
2-Ethyl-4-methyl-1H-imidazole is a useful synthetic intermediate. It can be used as a building block for active ingredients as well as in epoxy curing. It is used as an intermediate for pharmaceuticals, agrochemicals, dyes, textile auxiliaries, pigments and other organic chemicals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Ethyl-4-methyl-1H-imidazole is a useful synthetic intermediate. It can be used as a building block for active ingredients as well as in epoxy curing. It is used as an intermediate for pharmaceuticals, agrochemicals, dyes, textile auxiliaries, pigments and other organic chemicals.
Solubility
Soluble in water (<210 g/1000 ml at 20°C).
Notes
Store in cool dry place in tightly closed container. With good ventilation. Store away from oxidizing agent.
2-Ethyl-4-methyl-1H-imidazole is a useful synthetic intermediate. It can be used as a building block for active ingredients as well as in epoxy curing. It is used as an intermediate for pharmaceuticals, agrochemicals, dyes, textile auxiliaries, pigments and other organic chemicals.
Solubility
Soluble in water (<210 g/1000 ml at 20°C).
Notes
Store in cool dry place in tightly closed container. With good ventilation. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- M. S. Heise; and G. C. Martin. Mechanism of 2-ethyl-4-methylimidazole in the curing of the diglycidyl ether of bisphenol A. Journal of Polymer Science Part C: Polymer Letters. 1988, 26 (3), 153-157,.
- John M. Barton; andPeter M. Shepherd. The curing reaction of an epoxide resin with 2-ethyl-4-methylimidazole, a calorimetric study of the kinetics of formation of epoxide-imidazole adducts. Die Makromolekulare Chemie. 1975, 176 (4), 919-930.
- Building block in synthesis of imidazoquinazolines as cardiovascular agents: J. Med. Chem., 34, 2671 (1991).