Search Thermo Fisher Scientific
Thermo Scientific Chemicals
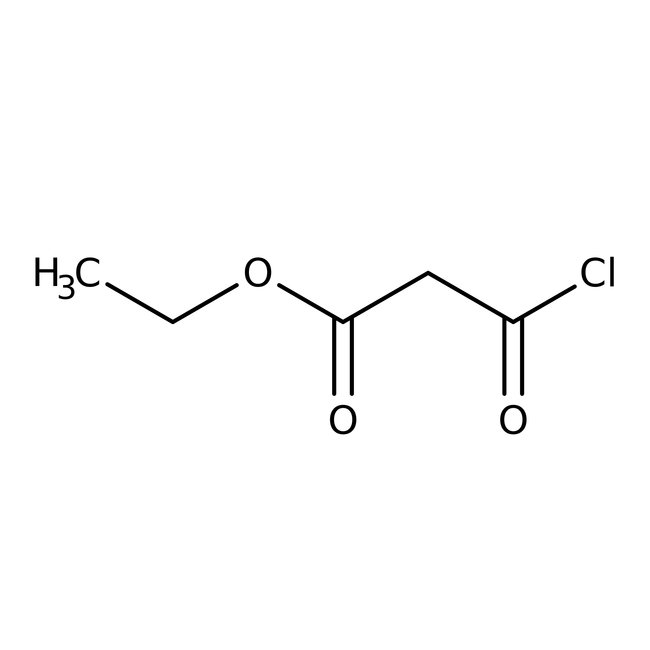
Ethyl malonyl chloride, 95%
CAS: 36239-09-5 | C5H7ClO3 | 150.56 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15616.06 | 5 g |
Catalog number ALFA15616.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or MaterialEthyl malonyl chloride
CAS36239-09-5
Health Hazard 1H227-H314-H335
Health Hazard 2GHS H Statement
H314-H318-H227
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
H314-H318-H227
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
Health Hazard 3P210-P235-P260-P264b-P271-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P370+P378q-P501c
View more
Ethyl malonyl chloride is used in the syntheses of methanofullerodendimers and 3-pyrrolin-2-ones. It plays a vital role in the preparation of 3,5-disubstituted 1,2,4-oxadiazole derivatives, which are potential peptidomimetic building blocks. It is a versatile acylating agent for propargyl alcohols, hydrazines and amines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl malonyl chloride is used in the syntheses of methanofullerodendimers and 3-pyrrolin-2-ones. It plays a vital role in the preparation of 3,5-disubstituted 1,2,4-oxadiazole derivatives, which are potential peptidomimetic building blocks. It is a versatile acylating agent for propargyl alcohols, hydrazines and amines.
Solubility
Miscible with water.
Notes
Store in cool place. Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with alcohols. It reacts with water.
Ethyl malonyl chloride is used in the syntheses of methanofullerodendimers and 3-pyrrolin-2-ones. It plays a vital role in the preparation of 3,5-disubstituted 1,2,4-oxadiazole derivatives, which are potential peptidomimetic building blocks. It is a versatile acylating agent for propargyl alcohols, hydrazines and amines.
Solubility
Miscible with water.
Notes
Store in cool place. Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with alcohols. It reacts with water.
RUO – Research Use Only
General References:
- Useful synthetic building block, e.g. in an oxazole synthesis: J. Med. Chem., 36, 3871 (1993):
- Formation of amides has been used in the syntheses of 1,2,4-triazine-3,6-diones, and of a vicinal tricarbonyl amide derivative of L-phenylalanine: J. Org. Chem., 60, 5992 (1995); 61, 1872 (1996).
- Yang, Z.; Li, S.; Zhang, Z.; Xu, J. Base-switched annuloselectivity in the reactions of ethyl malonyl chloride and imines. Org. Biomol. Chem. 2014, 12 (48), 9822-9830.
- Hayat, F.; Kang, L.; Lee, C. Y.; Shin, D. Synthesis of arylnaphthalene lignan lactone using benzoin condensation, intramolecular thermal cyclization and Suzuki coupling. Tetrahedron 2015, 71 (19), 2945-2950.