Search Thermo Fisher Scientific
Thermo Scientific Chemicals
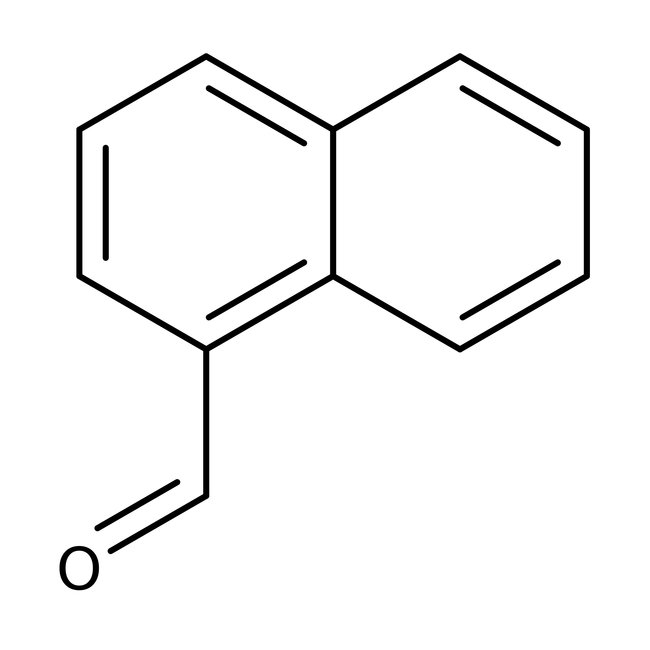
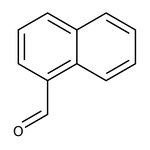
1-Naphthaldehyde, 97%
CAS: 66-77-3 | C11H8O | 156.18 g/mol
Catalog number ALFA15610.30
Price (MYR)
1,123.00
EA
Quantity:
250 g
Price (MYR)
1,123.00
EA
Specifications
Chemical Name or Material1-Naphthaldehyde
CAS66-77-3
Health Hazard 1H302-H312-H315-H319-H332-H335
Health Hazard 2GHS H Statement
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
1-Naphthaldehyde is used in the synthesis of single-crystalline homochiral porous metal-organic frameworks (MOFs). It is also a starting material for the Canizzaro reaction to produce 1-Naphthoic acid and 1-Naphthalenemethanol.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Naphthaldehyde is used in the synthesis of single-crystalline homochiral porous metal-organic frameworks (MOFs). It is also a starting material for the Canizzaro reaction to produce 1-Naphthoic acid and 1-Naphthalenemethanol.
Solubility
Soluble in ethanol, ether, acetone, benzene. Insoluble in water.
Notes
Air sensitive. Store under inert gas. Store away from oxidizing agent, air.
1-Naphthaldehyde is used in the synthesis of single-crystalline homochiral porous metal-organic frameworks (MOFs). It is also a starting material for the Canizzaro reaction to produce 1-Naphthoic acid and 1-Naphthalenemethanol.
Solubility
Soluble in ethanol, ether, acetone, benzene. Insoluble in water.
Notes
Air sensitive. Store under inert gas. Store away from oxidizing agent, air.
RUO – Research Use Only
General References:
- K Wichmann; T Krusius; R Sinervirta; J Puranen; J Jänne. Studies on structure-activity relationship of gossypol, gossypol ethers and three naphthaldehydes in the inhibition of spermatozoal metabolism. Contraception. 1986,35 (5), 519-528.
- Jerry A. Bell; Henry. Linschitz. Decay Kinetics of the 1-Naphthaldehyde and Benzophenone Triplet States in Benzene. J. Am. Chem. Soc. 1963, 85 (5), 528-533.