Search Thermo Fisher Scientific
Thermo Scientific Chemicals
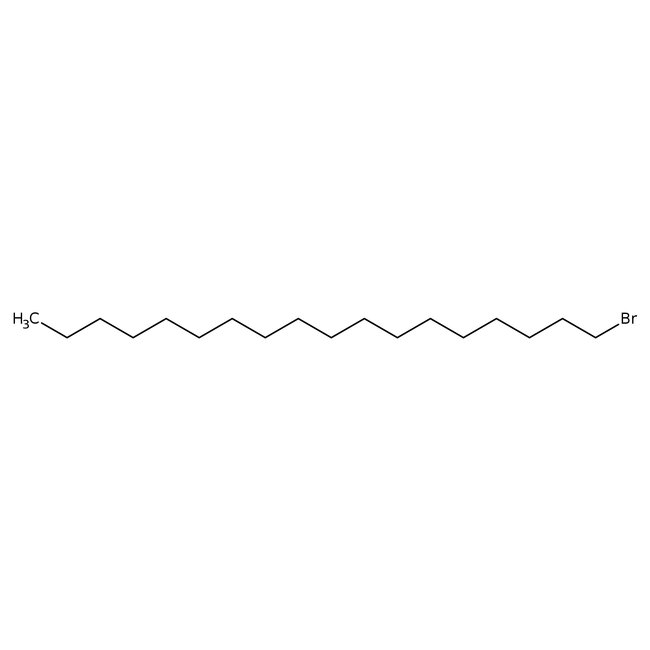
1-Bromooctadecane, 97%
CAS: 112-89-0 | C18H37Br | 333.40 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15588.22 | 100 g |
Catalog number ALFA15588.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material1-Bromooctadecane
CAS112-89-0
Melting Point25°C to 30°C
Recommended StorageAmbient temperatures
Density0.976
View more
1-Bromooctadecane is used in the preparation of shortened single-walled carbon nanotubes (s-SWCNTs). It is utilized to prepare octadecane in the presence of sodium borohydride as a catalyst. It is involved as a raw material for the preparation of dimethyldistearylammonium bromide, which is a bentonite modifier.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Bromooctadecane is used in the preparation of shortened single-walled carbon nanotubes (s-SWCNTs). It is utilized to prepare octadecane in the presence of sodium borohydride as a catalyst. It is involved as a raw material for the preparation of dimethyldistearylammonium bromide, which is a bentonite modifier.
Solubility
Soluble in acetone and most common organic solvents. Insoluble in water.
Notes
Incompatible with strong oxidizing agents and strong bases. Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place.
1-Bromooctadecane is used in the preparation of shortened single-walled carbon nanotubes (s-SWCNTs). It is utilized to prepare octadecane in the presence of sodium borohydride as a catalyst. It is involved as a raw material for the preparation of dimethyldistearylammonium bromide, which is a bentonite modifier.
Solubility
Soluble in acetone and most common organic solvents. Insoluble in water.
Notes
Incompatible with strong oxidizing agents and strong bases. Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place.
RUO – Research Use Only
General References:
- Aviv, H.; Tischler, Y. R. Synthesis and characterization of a J-aggregating TDBC derivative in solution and in Langmuir-Blodgett films. J. Lumin. 2015, 158, 376-383.
- Micewicz, E. D.; Ratikan, J. A.; Waring, A. J.; Whitelegge, J. P.; McBride, W. H.; Ruchala, P. Lipid-conjugated Smac analogues. Bioorg. Med. Chem. Lett. 2015, 25 (20), 4419-4427.