Search Thermo Fisher Scientific
Thermo Scientific Chemicals
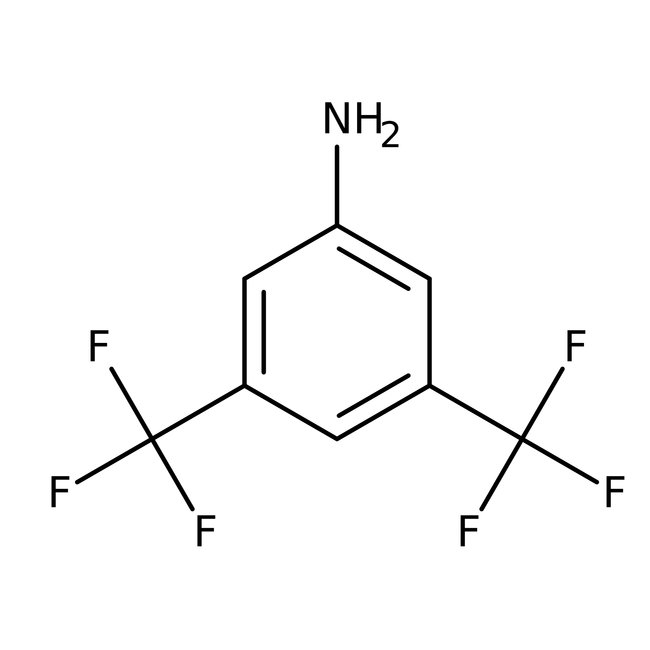
3,5-Bis(trifluoromethyl)aniline, 98%
CAS: 328-74-5 | C8H5F6N | 229.125 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15289.18 | 50 g |
Catalog number ALFA15289.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Specifications
Chemical Name or Material3,5-Bis(trifluoromethyl)aniline
CAS328-74-5
Health Hazard 1H227-H302+H312+H332-H315-H319-H335-H373
Health Hazard 2GHS H Statement
H331-H373-H227-H302-H312-H315-H319
Toxic if inhaled.
May cause damage to organs through prolonged or repeated exposure.
Combustible liquid.
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
H331-H373-H227-H302-H312-H315-H319
Toxic if inhaled.
May cause damage to organs through prolonged or repeated exposure.
Combustible liquid.
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P210-P235-P260-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P314-P330-P332+P313-P362-P370+P378q-P501c
View more
3,5-Bis(trifluoromethyl)aniline was used in the synthesis of N-[2-hydroxy-1-naphthylidene]-3, 5-bis(trifluoromethyl)aniline, Schiff?s base and 5,7-bis(trifluoromethyl)aniline. It was also used in the synthesis of N-1-phenylethyl-3,5-bis(trifluoromethyl)aniline via titanium-catalyzed hydroamination reaction and N,N?-bis[3,5-bis(tri-fluoromethyl)phenyl]thiourea, organocatalyst.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3,5-Bis(trifluoromethyl)aniline was used in the synthesis of N-[2-hydroxy-1-naphthylidene]-3, 5-bis(trifluoromethyl)aniline, Schiff′s base and 5,7-bis(trifluoromethyl)aniline. It was also used in the synthesis of N-1-phenylethyl-3,5-bis(trifluoromethyl)aniline via titanium-catalyzed hydroamination reaction and N,N′-bis[3,5-bis(tri-fluoromethyl)phenyl]thiourea, organocatalyst.
Solubility
Not miscible in water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
3,5-Bis(trifluoromethyl)aniline was used in the synthesis of N-[2-hydroxy-1-naphthylidene]-3, 5-bis(trifluoromethyl)aniline, Schiff′s base and 5,7-bis(trifluoromethyl)aniline. It was also used in the synthesis of N-1-phenylethyl-3,5-bis(trifluoromethyl)aniline via titanium-catalyzed hydroamination reaction and N,N′-bis[3,5-bis(tri-fluoromethyl)phenyl]thiourea, organocatalyst.
Solubility
Not miscible in water.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Ludwig T.Kaspar; Benjamin Fingerhut; Lutz Ackermann. Titanium-catalyzed intermolecular hydroamination of vinylarene. Angewandte Chemie (International Edition). 2005, 44,(37), 5972-5974.
- Yunoh Jung; Kang-Jun Baeg; Dong-Yu Kim; Takao Someya; Soo Young Park. A thermally resistant and air-stable n-type organic semiconductor: Naphthalene diimide of 3,5-bis-trifluoromethyl aniline. Synthetic Metals. 2009, 159,(19-20), 2117-2121.