Search Thermo Fisher Scientific
Thermo Scientific Chemicals
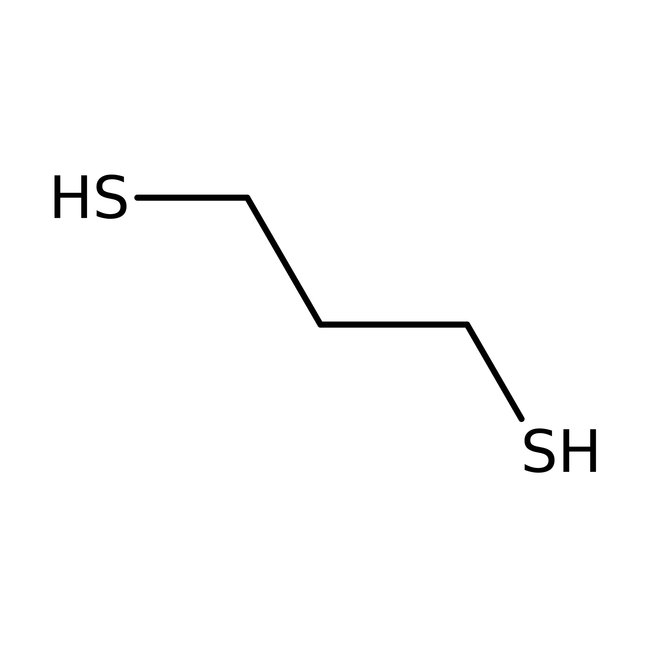
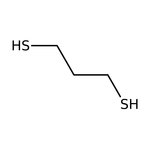
1,3-Propanedithiol, 97%
CAS: 109-80-8 | C3H8S2 | 108.217 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15261.14 | 25 g |
Catalog number ALFA15261.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material1,3-Propanedithiol
CAS109-80-8
Health Hazard 1H226-H301-H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P270-P271-P280-P301+P310-P303+P361+P353-P304+P340-P305+P351+P338-P312-P330-P332+P313-P363-P370+P378q-P501c
View more
1,3-Propanedithiol is used as a reagent in the preparation of thioketals and thioacetals. It acts as a flavoring agent. It is used as a precursor in the synthesis of cyclic dithioacetal (1,3-dithiane) derivatives of carbonyl compounds. It is involved in the preparation of diiron propanedithiolate hexacarbonyl by reacting reaction with triiron dodecacarbonyl. Further, it is used for the protection of aldehydes and ketones through their reversible formation of dithianes. In addition to this, it reacts with metal ions to form chelate rings.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3-Propanedithiol is used as a reagent in the preparation of thioketals and thioacetals. It acts as a flavoring agent. It is used as a precursor in the synthesis of cyclic dithioacetal (1,3-dithiane) derivatives of carbonyl compounds. It is involved in the preparation of diiron propanedithiolate hexacarbonyl by reacting reaction with triiron dodecacarbonyl. Further, it is used for the protection of aldehydes and ketones through their reversible formation of dithianes. In addition to this, it reacts with metal ions to form chelate rings.
Solubility
Slightly soluble in water. Miscible with alcohol, ether, chloroform, etherMiscible with alcohol, ether, chloroform and ether. Slightly miscible with water.
Notes
Store in a cool place. Incompatible with bases, oxidizing agents, reducing agents and alkali metals.
1,3-Propanedithiol is used as a reagent in the preparation of thioketals and thioacetals. It acts as a flavoring agent. It is used as a precursor in the synthesis of cyclic dithioacetal (1,3-dithiane) derivatives of carbonyl compounds. It is involved in the preparation of diiron propanedithiolate hexacarbonyl by reacting reaction with triiron dodecacarbonyl. Further, it is used for the protection of aldehydes and ketones through their reversible formation of dithianes. In addition to this, it reacts with metal ions to form chelate rings.
Solubility
Slightly soluble in water. Miscible with alcohol, ether, chloroform, etherMiscible with alcohol, ether, chloroform and ether. Slightly miscible with water.
Notes
Store in a cool place. Incompatible with bases, oxidizing agents, reducing agents and alkali metals.
RUO – Research Use Only
General References:
- Precursor of cyclic dithioacetal (1,3-dithiane) derivatives of carbonyl compounds. The protection step is catalyzed by Lewis acids, e.g. BF3 etherate: Tetrahedron Lett., 871 (1971), TiCl4: Tetrahedron Lett., 24, 1289 (1983), Aluminum trifluoromethanesulfonate, B20785, or Indium(III) trifluoromethanesulfonate, 40131. Diothioacetalization can be accomplished under neutral, solvent-free conditions using Lithium trifluoromethanesulfonate, 39322, as catalyst: Tetrahedron Lett., 40, 4055 (1999). For the preparation of monocyclic dithioacetals of ß-diketones at low temperature in the presence of BF3 etherate, see: Tetrahedron, 44, 2283 (1988).
- Alternatively, 1,3-dithianes can be prepared by alkylation with reactive gem-dihalides under phase-transfer conditions: Liebigs Ann. Chem., 1589 (1982).
- For deprotection methods and uses of 1,3-dithianes as acyl anion equivalents, see: 1,3-Dithiane, A10505.
- Wang, L.; Li, R.; Feng, L.; Liu, J.; Gao, X.; Wang, W. Study on the interface electronic states of chemically modified ZnO nanowires. RSC Adv. 2015, 5 (119), 98130-98135.
- Kuciński, K.; Pawluć, P.; Marciniec, B.; Hreczycho, G. Highly Selective Hydrothiolation of Unsaturated Organosilicon Compounds Catalyzed by Scandium(III) Triflate. Chem. Eur. J. 2015, 21 (13), 4940-4943.