Search Thermo Fisher Scientific
Thermo Scientific Chemicals
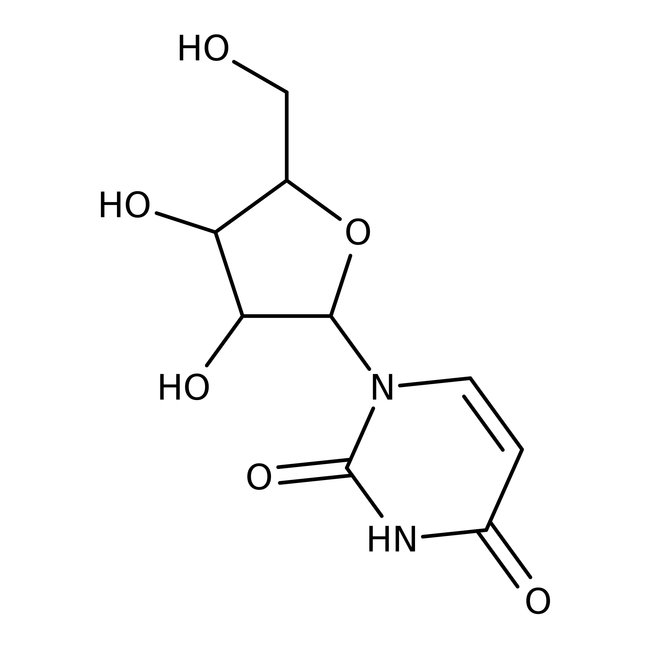
Uridine, 99%
Uridine, 99%, C9H12N2O6, CAS Number-58-96-8, uracil, 1-beta-d-ribofuranosyl, uracil riboside, d-uridine, beta-uridine, 1-beta-d-ribofuranosyluracil, uridin, unii-whi7hq7h85, urd, b-uridine, uridine, 100g, 754904, 200-407-5, 244.2, DRTQHJPVMGBUCF-DHRBYNEYSA-N | CAS: 58-96-8 | C9H12N2O6 | 244.203 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15227.06 | 5 g |
Catalog number ALFA15227.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or MaterialUridine
CAS58-96-8
Melting Point166°C to 169°C
Recommended StorageAmbient temperatures
RTECS NumberYR1450000
View more
Uridine plays a vital role in the glycolysis pathway of galactose. It is used as a precursor in the production of CDP-choline. It is an important nutrient and widely used as a dietary supplement. It improves brain cholinergic functions and hepatic mitochondrial function in certain liver toxins. It plays a major role in pain physiology and brain energy utilization to maintain ATP production under restricted oxygen conditions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Uridine plays a vital role in the glycolysis pathway of galactose. It is used as a precursor in the production of CDP-choline. It is an important nutrient and widely used as a dietary supplement. It improves brain cholinergic functions and hepatic mitochondrial function in certain liver toxins. It plays a major role in pain physiology and brain energy utilization to maintain ATP production under restricted oxygen conditions.
Solubility
Soluble in water, dimethylsulfoxide, and methanol.
Notes
Store in a cool place. Incompatible with strong oxidizing agents.
Uridine plays a vital role in the glycolysis pathway of galactose. It is used as a precursor in the production of CDP-choline. It is an important nutrient and widely used as a dietary supplement. It improves brain cholinergic functions and hepatic mitochondrial function in certain liver toxins. It plays a major role in pain physiology and brain energy utilization to maintain ATP production under restricted oxygen conditions.
Solubility
Soluble in water, dimethylsulfoxide, and methanol.
Notes
Store in a cool place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Heuberger, B. D.; Pal, A.; Del Frate, F.; Topkar, V. V.; Szostak, J. W. Replacing Uridine with 2-Thiouridine Enhances the Rate and Fidelity of Nonenzymatic RNA Primer Extension. J. Am. Chem. Soc. 2015, 137 (7), 2769-2775.
- L Ipata, P.; Pesi, R. Metabolic Regulation of Uridine in the Brain. Curr Metabolomics 2015, 3 (1), 4-9.