Search Thermo Fisher Scientific
Thermo Scientific Chemicals
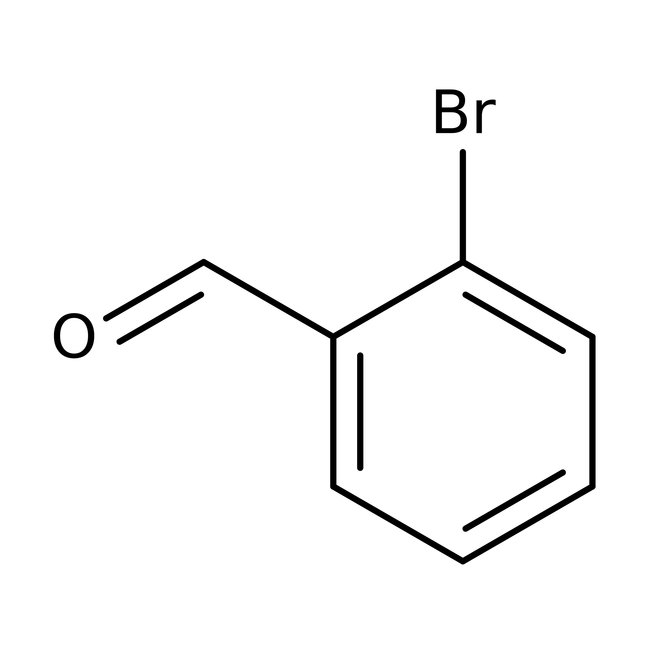
2-Bromobenzaldehyde, 98%
CAS: 6630-33-7 | C7H5BrO | 185.02 g/mol
Catalog number ALFA15065.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material2-Bromobenzaldehyde
CAS6630-33-7
Health Hazard 1H302-H315-H319-H335
Health Hazard 2GHS H Statement
H302-H315-H319
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
H302-H315-H319
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
2-Bromobenzaldehyde is used in L-threonine aldolase-catalyzed enantio and diastereoselective aldol reactions. Further, it reacts with trichloromethane to prepare 1-(2-bromo-phenyl)-2,2,2-trichloro-ethanol.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Bromobenzaldehyde is used in L-threonine aldolase-catalyzed enantio and diastereoselective aldol reactions. Further, it reacts with trichloromethane to prepare 1-(2-bromo-phenyl)-2,2,2-trichloro-ethanol.
Solubility
Soluble in alcohol and benzene. Insoluble in water.
Notes
Air sensitive. Incompatible with strong bases, strong oxidizing agents and strong reducing agents.
2-Bromobenzaldehyde is used in L-threonine aldolase-catalyzed enantio and diastereoselective aldol reactions. Further, it reacts with trichloromethane to prepare 1-(2-bromo-phenyl)-2,2,2-trichloro-ethanol.
Solubility
Soluble in alcohol and benzene. Insoluble in water.
Notes
Air sensitive. Incompatible with strong bases, strong oxidizing agents and strong reducing agents.
RUO – Research Use Only
General References:
- Vinoth, P.; Vivekanand, T.; Suryavanshi, P. A.; Menéndez, J. C.; Sasai, H.; Sridharan, V. Palladium(II)-catalyzed intramolecular carboxypalladation-olefin insertion cascade: direct access to indeno[1,2-b]furan-2-ones. Org. Biomol. Chem. 2015, 13 (18), 5175-5181.
- Saoudi, B.; Teniou, A.; Debache, A.; Roisnel, T.; Soulé, J. F.; Doucet, H. Cyclisation reaction between 3-methylquinoxaline-2-thione and benzaldehydes into 3-benzyl-2-aryl-thieno[2,3-b]quinoxaline promoted by Brønsted acids. C.R. Chim. 2015, 18 (8), 808-815.