Search Thermo Fisher Scientific
Thermo Scientific Chemicals
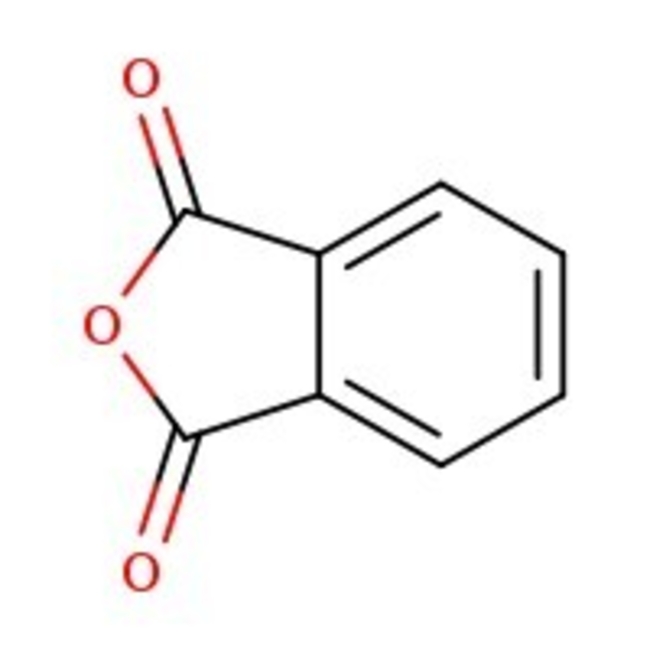
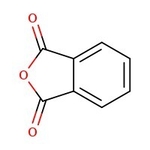
Phthalic anhydride, 99%
CAS: 85-44-9 | C8H4O3 | 148.12 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14955.0B | 1000 g |
Catalog number ALFA14955.0B
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1000 g
Specifications
Chemical Name or MaterialPhthalic anhydride
CAS85-44-9
Health Hazard 1H302-H315-H317-H318-H334-H335-H500
Health Hazard 2GHS H Statement
H334-H318-H302-H335-H315-H317
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
Causes serious eye damage.
Harmful if swallowed.
May cause respiratory irritation.
Causes skin irritation.
May cause an allergic skin reaction.
H334-H318-H302-H335-H315-H317
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
Causes serious eye damage.
Harmful if swallowed.
May cause respiratory irritation.
Causes skin irritation.
May cause an allergic skin reaction.
Health Hazard 3P261-P264b-P270-P271-P272-P280-P285-P301+P312-P302+P352-P304+P340-P305+P351+P338-P310-P330-P333+P313-P342+P311-P362-P501c
View more
Phthalic anhydride is used in the preparation of primary amines. It is used as a precursor to prepare anthraquinone, phthalein, rhodamine, phthalocyanine, fluorescein and xanthene dyes. It serves as an important intermediate in the plastics industry, dyes, resins and pigments. It is used as a monomer for synthetic resins such as glyptal, alkyd resins and polyester resins. It plays a vital role in the large-scale production of plasticizers for plastics. Further, it acts as a curing agent for epoxy resins, anti-scaling agents, plating agents, surface treating agents, ion exchange agents, paint additives, adhesives, sealant chemicals and potential antibacterial agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phthalic anhydride is used in the preparation of primary amines. It is used as a precursor to prepare anthraquinone, phthalein, rhodamine, phthalocyanine, fluorescein and xanthene dyes. It serves as an important intermediate in the plastics industry, dyes, resins and pigments. It is used as a monomer for synthetic resins such as glyptal, alkyd resins and polyester resins. It plays a vital role in the large-scale production of plasticizers for plastics. Further, it acts as a curing agent for epoxy resins, anti-scaling agents, plating agents, surface treating agents, ion exchange agents, paint additives, adhesives, sealant chemicals and potential antibacterial agent.
Solubility
Soluble in water, carbon disulfide, ethanol, acetone and benzene. Slightly soluble in ethyl ether.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents, strong bases, strong reducing agents and strong acids.
Phthalic anhydride is used in the preparation of primary amines. It is used as a precursor to prepare anthraquinone, phthalein, rhodamine, phthalocyanine, fluorescein and xanthene dyes. It serves as an important intermediate in the plastics industry, dyes, resins and pigments. It is used as a monomer for synthetic resins such as glyptal, alkyd resins and polyester resins. It plays a vital role in the large-scale production of plasticizers for plastics. Further, it acts as a curing agent for epoxy resins, anti-scaling agents, plating agents, surface treating agents, ion exchange agents, paint additives, adhesives, sealant chemicals and potential antibacterial agent.
Solubility
Soluble in water, carbon disulfide, ethanol, acetone and benzene. Slightly soluble in ethyl ether.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents, strong bases, strong reducing agents and strong acids.
RUO – Research Use Only
General References:
- For examples of formation of phthalimides from amines, see: Org. Synth. Coll., 4, 106 (1963); 5, 973 (1973). As an alternative to hydrazinolysis, ɑ-amino acids can also be quantitatively recovered from their phthaloyl derivatives by prolonged reaction with aqueous methylamine at room temperature: Can. J. Chem., 48, 3572 (1970).
- Can be used for the dehydration of nitro aldols to nitroalkenes; see, e.g.: Org. Synth. Coll., 7, 396 (1990).
- Reaction with H2O2 gives monoperphthalic acid, useful in peroxidation reactions; see, e.g.: Org. Synth. Coll., 3, 619 (1955); 5, 805 (1973). With urea hydrogen peroxide, provides a superior system for converting N-heteroaromatics and tert-amines to their N-oxides: Chem. Ber., 125, 1965 (1992)
- Liu, Y.; Gu, Y.; Hou, Y.; Yang, Y.; Deng, S.; Wei, Z. Hydrophobic activated carbon supported Ni-based acid-resistant catalyst for selective hydrogenation of phthalic anhydride to phthalide. Chem. Eng. J. 2015, 275, 271-280.
- Lin, Z.; Ierapetritou, M.; Nikolakis, V. Phthalic anhydride production from hemicellulose solutions: Technoeconomic analysis and life cycle assessment. AlChE J. 2015, 61 (11), 3708-3718.