Search Thermo Fisher Scientific
Thermo Scientific Chemicals
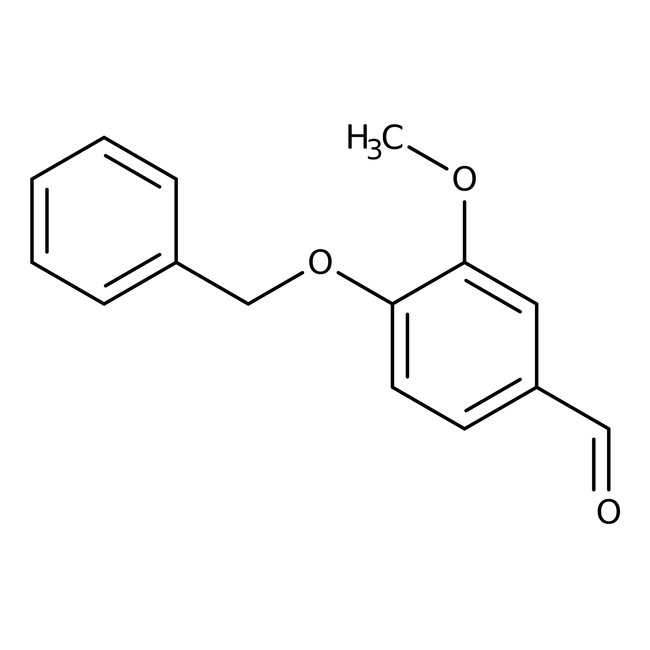
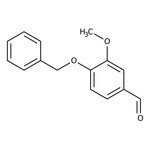
4-Benzyloxy-3-methoxybenzaldehyde, 98%
CAS: 2426-87-1 | C15H14O3 | 242.27 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14716.14 | 25 g |
Catalog number ALFA14716.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material4-Benzyloxy-3-methoxybenzaldehyde
CAS2426-87-1
Health Hazard 1Warning
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3GHS P Statement
P261-P280-P305+P351+P338-P304+P340-P405-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
P261-P280-P305+P351+P338-P304+P340-P405-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
View more
4-Benzyloxy-3-methoxybenzaldehyde was used in the synthesis of 1,2-bis(4-benzyloxy-3-methoxyphenyl)-3-hydroxy-propionic acid1. It was also used in first enantioselective total synthesis of a neurotrophic (-)-talaumidin. 4-Benzyloxy-3-methoxybenzaldehyde reacts with benzohydrazide to yield (E)-N?-(4-benzyloxy-3-methoxybenzylidene)benzohydrazide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Benzyloxy-3-methoxybenzaldehyde was used in the synthesis of 1,2-bis(4-benzyloxy-3-methoxyphenyl)-3-hydroxy-propionic acid1. It was also used in first enantioselective total synthesis of a neurotrophic (-)-talaumidin. 4-Benzyloxy-3-methoxybenzaldehyde reacts with benzohydrazide to yield (E)-N′-(4-benzyloxy-3-methoxybenzylidene)benzohydrazide.
Solubility
Soluble in chloroform, methanol.
Notes
Air Sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from strong oxidizing agents and strong bases.
4-Benzyloxy-3-methoxybenzaldehyde was used in the synthesis of 1,2-bis(4-benzyloxy-3-methoxyphenyl)-3-hydroxy-propionic acid1. It was also used in first enantioselective total synthesis of a neurotrophic (-)-talaumidin. 4-Benzyloxy-3-methoxybenzaldehyde reacts with benzohydrazide to yield (E)-N′-(4-benzyloxy-3-methoxybenzylidene)benzohydrazide.
Solubility
Soluble in chloroform, methanol.
Notes
Air Sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- J Shi. (E)-N'-(4-Benzyloxy-3-methoxybenzylidene) isonicotinohydrazide. Acta Crystallographica Section E: Structure Reports. 2005E61 (2), 3933-3934.
- T Hashimoto.; K Hasegawa.; H Yamaguchi.; M Saito. Structure and synthesis of batatasins, dormancy-inducing substances of yam bulbils. Phytochemistry. 197413 (12), 2849-2852.