Search Thermo Fisher Scientific
Thermo Scientific Chemicals
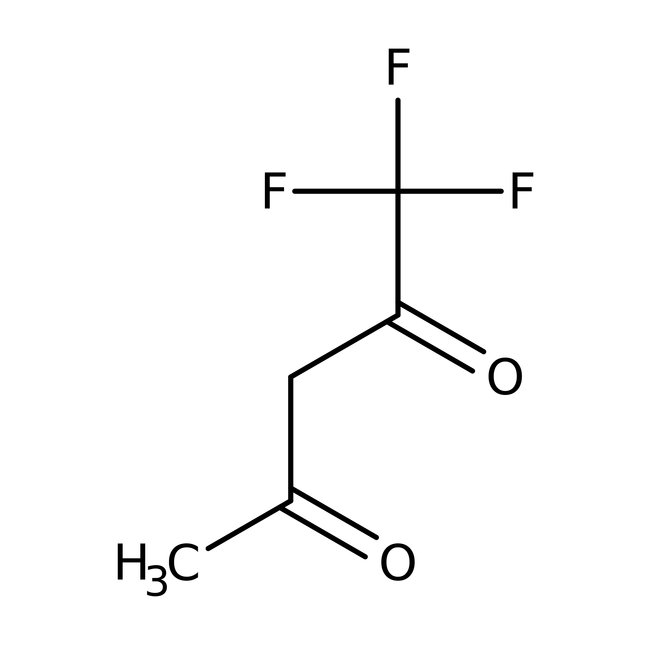
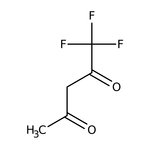
1,1,1-Trifluoro-2,4-pentanedione, 98%
CAS: 367-57-7 | C5H5F3O2 | 154.09 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14682.06 | 5 g |
Catalog number ALFA14682.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or Material1,1,1-Trifluoro-2,4-pentanedione
CAS367-57-7
Health Hazard 1H226-H302+H312+H332-H315-H319-H335
Health Hazard 2GHS H Statement
H301-H226-H315-H319-H335
Toxic if swallowed.
Flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H301-H226-H315-H319-H335
Toxic if swallowed.
Flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P270-P271-P280-P301+P312-P303+P361+P353-P304+P340-P305+P351+P338-P312-P330-P332+P313-P363-P370+P378q-P501c
View more
1,1,1-Trifluoro-2,4-pentanedione has been used as reagent in the preparation of 2-alkylcarbonyl and 2-benzoyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,1,1-Trifluoro-2,4-pentanedione has been used as reagent in the preparation of 2-alkylcarbonyl and 2-benzoyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives.
Solubility
Soluble in water (slightly) and many organic solvents.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
1,1,1-Trifluoro-2,4-pentanedione has been used as reagent in the preparation of 2-alkylcarbonyl and 2-benzoyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives.
Solubility
Soluble in water (slightly) and many organic solvents.
Notes
Stable under recommended storage conditions. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Belen Zarranz; Andres Jaso; Ignacio Aldana; Antonio Monge. Synthesis and anticancer activity evaluation of new 2-alkylcarbonyl and 2-benzoyl-3-trifluoromethyl-quinoxaline 1,4-di-N-oxide derivatives. Bioorganic & Medicinal Chemistry. 2004, 12, (13),3711-3721
- Mansoureh Zahedi-Tabrizi; Fariba Tayyari; Zainab Moosavi-Tekyeh; Alireza Jalali; Sayyed Faramarz Tayyari. Structure and vibrational assignment of the enol form of 1,1,1-trifluoro-2,4-pentanedione. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2006, 65, (2),387-396
- Chelating ligand. Review of chemistry of fluorinated ß-diketones: Russ. Chem. Rev., 50, 180 (1981).
- For use in the preparation of trifluoromethyl pyrimidines by reaction with guanidines, see: Synth. Commun., 20, 913 (1990):