Search Thermo Fisher Scientific
Thermo Scientific Chemicals
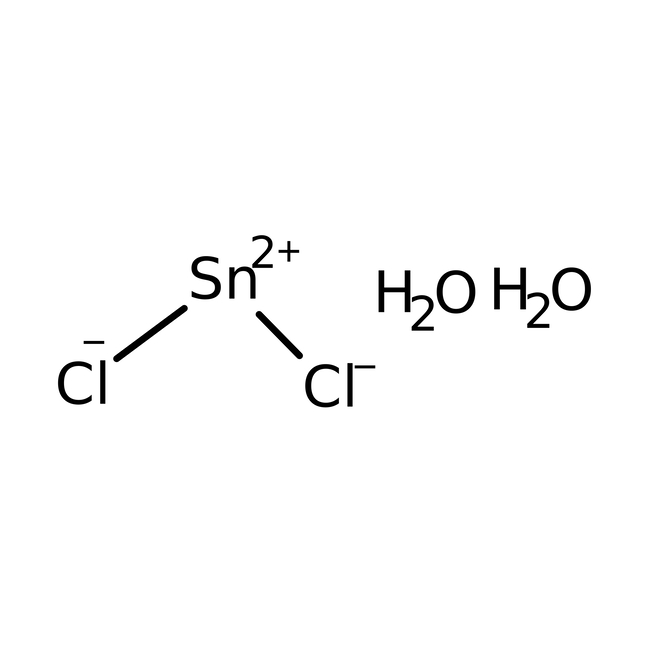
Tin(II) chloride dihydrate, 98%
CAS: 10025-69-1 | Cl2Sn·2H2O
Catalog number ALFA14610.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialTin(II) chloride dihydrate
Melting Point42°C to 46°C
CAS10025-69-1
Health Hazard 1H290-H314-H317-H332-H335-H373
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
View more
Tin(II) chloride is used as a sensitizing agent in preparing glass and plastic for metalizing. It serves as a potent reducing agent. Other uses of stannous chloride are tin electroplating baths, corrosion inhibitors, polymers, thermoplastic elastomers, soldering flux, antioxidant, tanning agent and pharmaceuticals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tin(II) chloride is used as a sensitizing agent in preparing glass and plastic for metalizing. It serves as a potent reducing agent. Other uses of stannous chloride are tin electroplating baths, corrosion inhibitors, polymers, thermoplastic elastomers, soldering flux, antioxidant, tanning agent and pharmaceuticals.
Solubility
Soluble in dilute and concentrated hydrochloric acid, alcohol, ethyl acetate, glacial acetic acid, and sodium hydroxide solution. Decomposes in excess water.
Notes
Hygroscopic, and absorbs moisture or water from air. Incompatible with metals, strong oxidizing agents, strong bases and bromine trifluoride.
Tin(II) chloride is used as a sensitizing agent in preparing glass and plastic for metalizing. It serves as a potent reducing agent. Other uses of stannous chloride are tin electroplating baths, corrosion inhibitors, polymers, thermoplastic elastomers, soldering flux, antioxidant, tanning agent and pharmaceuticals.
Solubility
Soluble in dilute and concentrated hydrochloric acid, alcohol, ethyl acetate, glacial acetic acid, and sodium hydroxide solution. Decomposes in excess water.
Notes
Hygroscopic, and absorbs moisture or water from air. Incompatible with metals, strong oxidizing agents, strong bases and bromine trifluoride.
RUO – Research Use Only
General References:
- Nitro aromatics can be selectively reduced in the presence of aldehyde, nitrile or halogen substituents, using ethanol - ethyl acetate as solvent: Tetrahedron Lett., 25, 839 (1984). ɑß-Unsaturated nitro compounds are reduced to the oximes of saturated ketones: Synth. Commun., 18, 693 (1988).
- Reduces azides to amines in methanol: Tetrahedron Lett., 27, 1425 (1986).
- For use in the preparation of aldehydes by the Stephen reduction of aromatic nitriles, see: Org. Synth. Coll., 3, 626 (1955), and by the Sonn-Mueller reduction of imidoyl chlorides: Org. Synth. Coll., 3, 818 (1955).
- Catalyzes the reaction of ethyl diazoacetate with aldehydes to give ß-keto esters: J. Org. Chem., 54, 3258 (1989). For carbon-carbon double-bond formation between ɑ-halo ketones and aldehydes, mediated by SnCl2a2SO3, see: Synth. Commun., 23, 271 (1993).
- Catalyst for tetrahydropyranylation of alcohols: Synth. Commun., 29, 1679 (1999).
- Gurnani, C.; Hector, A. L.; Jager, E.; Levason, W.; Pugha, D.; Reid, G. Tin(II) fluoride vs. tin(II) chloride - a comparison of their coordination chemistry with neutral ligands. Dalton Trans. 2013, 42, 8364-8374.
- Ramasamy, K.; Malika, M. A.; O'Brien, P. Routes to copper zinc tin sulfide Cu2ZnSnS4 a potential material for solar cells. Chem. Commun. 2012, 48, 5703-5714.

.png-150.jpg)
