Search Thermo Fisher Scientific
Thermo Scientific Chemicals
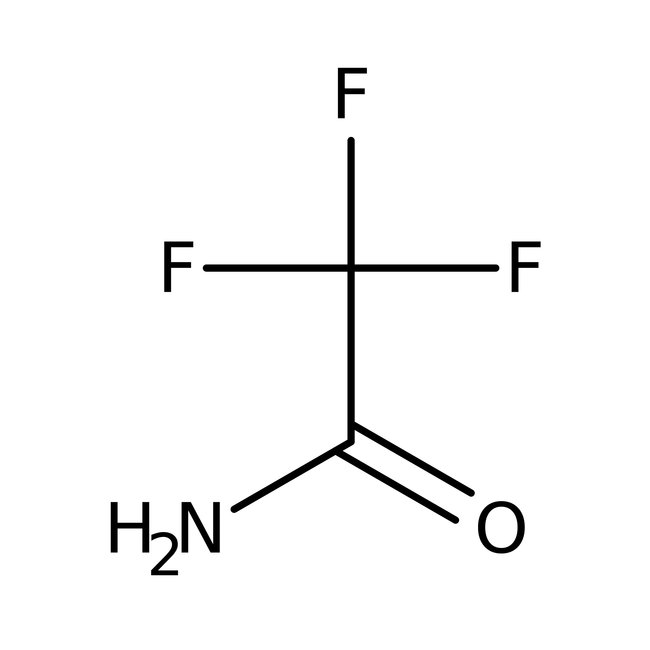
2,2,2-Trifluoroacetamide, 97%
CAS: 354-38-1 | C2H2F3NO | 113.04 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14451.22 | 100 g |
Catalog number ALFA14451.22
Price (MYR)
876.00
EA
Quantity:
100 g
Price (MYR)
876.00
EA
Specifications
Chemical Name or Material2,2,2-Trifluoroacetamide
CAS354-38-1
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
2,2,2-Trifluoroacetamide used in a convenient alternative to the Gabriel synthesis of primary amines from halides by N-alkylation followed by cleavage of the readily-hydrolyzed trifluoroacetyl group and for improved N-alkylation under phase-transfer conditions allowing successive alkylation with different alkyl groups.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,2,2-Trifluoroacetamide used in a convenient alternative to the Gabriel synthesis of primary amines from halides by N-alkylation followed by cleavage of the readily-hydrolyzed trifluoroacetyl group and for improved N-alkylation under phase-transfer conditions allowing successive alkylation with different alkyl groups.
Solubility
Soluble in water (460 g/L at 20°C), alcohol, ether, chloroform (partially), and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, strong acids, strong bases and strong reducing agents.
2,2,2-Trifluoroacetamide used in a convenient alternative to the Gabriel synthesis of primary amines from halides by N-alkylation followed by cleavage of the readily-hydrolyzed trifluoroacetyl group and for improved N-alkylation under phase-transfer conditions allowing successive alkylation with different alkyl groups.
Solubility
Soluble in water (460 g/L at 20°C), alcohol, ether, chloroform (partially), and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, strong acids, strong bases and strong reducing agents.
RUO – Research Use Only
General References:
- Chuan-Zhou Tao.; Juan Li.; Yao Fu.; Lei Liu.; Qing-Xiang Guo. Copper-catalyzed synthesis of primary arylamines from aryl halides and 2,2,2-trifluoroacetamide. Tetrahedron Letters. 2008, 49 (1), 70-75.
- Snefrid Gundersen.; Svein Samdal.; Ragnhild Seip.; Dmitry J. Shorokhov.; Tor G. Strand. Applequist. The molecular structure, conformation, potential to internal rotation and force field of 2,2,2-trifluoroacetamide as studied by gas electron diffraction and quantum chemical calculations. Journal of Molecular Structure. 1998, 445 (1-3), 229-242.
- Has been used in a convenient alternative to the Gabriel synthesis of primary amines from halides by N-alkylation followed by cleavage of the readily-hydrolyzed trifluoroacetyl group: Synthesis, 941 (1984). For improved N-alkylation under phase-transfer conditions allowing successive alkylation with different alkyl groups, see: Synth. Commun., 18, 791 (1988). The method has been further extended by the use of ɑ-bromo esters to provide a high yielding route to ɑ-amino acids: J. Org. Chem., 56, 420 (1991):