Search Thermo Fisher Scientific
Thermo Scientific Chemicals
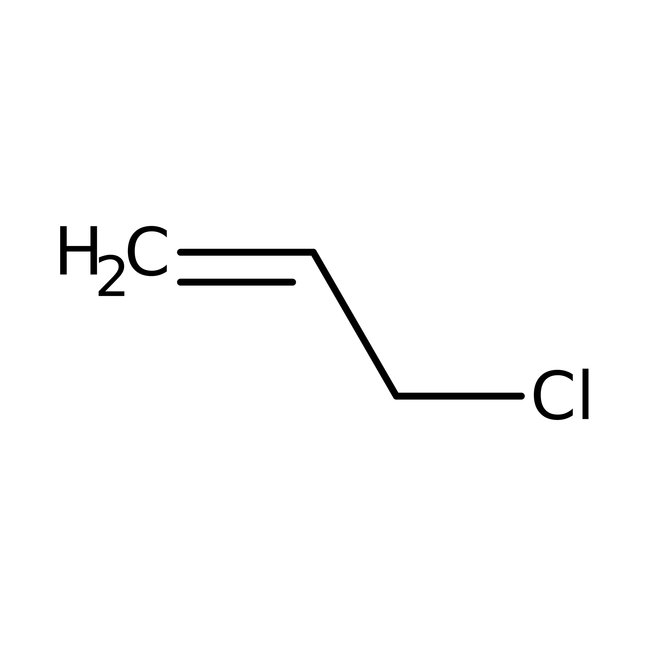
Allyl chloride, 98%, stab. with propylene oxide, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFA14330.AP | 500 mL |
Catalog number ALFA14330.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or MaterialAllyl chloride
Name Notestabilized with propylene oxide
CAS107-05-1
Health Hazard 1H225-H302+H312+H332-H315-H319-H335-H341-H351-H373
Health Hazard 2GHS H Statement
H225-H341-H351-H373-H302-H312-H332-H315-H319-H335
Highly flammable liquid and vapour.
Suspected of causing genetic defects.
Suspected of causing cancer.
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H225-H341-H351-H373-H302-H312-H332-H315-H319-H335
Highly flammable liquid and vapour.
Suspected of causing genetic defects.
Suspected of causing cancer.
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
Allyl chloride is used as an alkylating agent. It is used in treatment with sodium bis(trimethylsilyl)amide gives the strained, highly reactive alkene cyclopropene.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Allyl chloride is used as an alkylating agent. It is used in treatment with sodium bis(trimethylsilyl)amide gives the strained, highly reactive alkene cyclopropene.
Solubility
Soluble in water (3600 mg/L at 20°C), ethanol, ether, petroleum, acetone, chloroform, benzene, methanol and 2-prop.
Notes
Store at -20°C. Keep tightly closed in a dry and cool place. Keep in properly labeled containers. Keep containers tightly closed in a cool, well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Incompatible with strong oxidizing agents, boron trifluoride, sulfuric acid and nitric acid
Allyl chloride is used as an alkylating agent. It is used in treatment with sodium bis(trimethylsilyl)amide gives the strained, highly reactive alkene cyclopropene.
Solubility
Soluble in water (3600 mg/L at 20°C), ethanol, ether, petroleum, acetone, chloroform, benzene, methanol and 2-prop.
Notes
Store at -20°C. Keep tightly closed in a dry and cool place. Keep in properly labeled containers. Keep containers tightly closed in a cool, well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Incompatible with strong oxidizing agents, boron trifluoride, sulfuric acid and nitric acid
RUO – Research Use Only
General References:
- Kenji Uneyama.; Nobuyoshi Kamaki.; Akihiko Moriya.; Sigeru Torii. Tin(II)-aluminum-promoted allylation of aldehydes with allyl chloride in an aqueous solvent system. J. Org. Chem. 1985, 50 (25), 5396-5399.
- Farley Fisher.; Douglas E. Applequist. Synthesis of 1-Methylcyclopropene. J. Org. Chem. 1965, 30 (6), 2089-2090.
- Lithiation with LDA in THF at -78° generatesɑ-chloroallyllithium which can be ɑ-alkylated with primary bromides in high yield. Displacement of the resulting allylic chloride with a dialkyl cuprate to give, stereoselectively, an (E)-alkene, has been used in a pheromone synthesis: J. Org. Chem., 45, 1504 (1981).
- Treatment with sodium bis(trimethylsilyl)amide gives the strained, highly reactive alkene cyclopropene. For details and Diels-Alder reactions, see: J. Org. Chem., 61, 6462 (1996).