Search Thermo Fisher Scientific
Thermo Scientific Chemicals
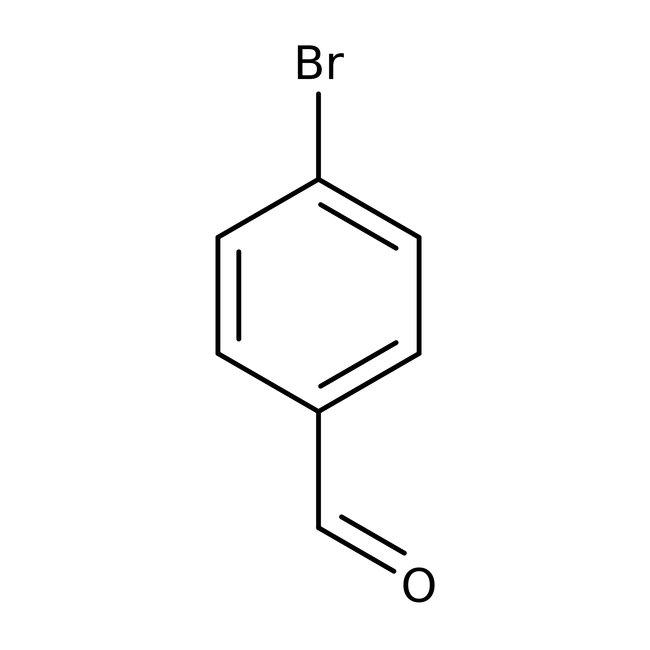
4-Bromobenzaldehyde, 98+%
CAS: 1122-91-4 | C7H5BrO | 185.02 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14237.14 | 25 g |
Catalog number ALFA14237.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material4-Bromobenzaldehyde
CAS1122-91-4
Health Hazard 1H302-H315-H319-H334-H335
Health Hazard 2GHS H Statement
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P285-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P342+P311-P362-P501c
View more
4-Bromobenzaldehyde is widely utilized as an intermediate for the preparation of agrochemicals, pharmaceuticals and other organic compounds. It is used as a precursor and reacts with sulfamethoxazole to prepare Schiff’s base, 4-[(4-Bromo-benzylidene)-amino]-N-(5-methyl-isoxazol-3-yl)-benzene sulfonamide, which can be used for the photo stability of polyvinyl chloride (PVC). It plays an important role in palladium-catalyzed mono- and bis-arylation of 3,4-(ethylenedioxy)thiophenes. It is employed in a cross-coupling study with potassium vinyltrifluoroborate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Bromobenzaldehyde is widely utilized as an intermediate for the preparation of agrochemicals, pharmaceuticals and other organic compounds. It is used as a precursor and reacts with sulfamethoxazole to prepare Schiff’s base, 4-[(4-Bromo-benzylidene)-amino]-N-(5-methyl-isoxazol-3-yl)-benzene sulfonamide, which can be used for the photo stability of polyvinyl chloride (PVC). It plays an important role in palladium-catalyzed mono- and bis-arylation of 3,4-(ethylenedioxy)thiophenes. It is employed in a cross-coupling study with potassium vinyltrifluoroborate.
Solubility
Soluble in ethanol and benzene. Insoluble in water.
Notes
Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong bases, strong oxidizing agents and strong reducing agents.
4-Bromobenzaldehyde is widely utilized as an intermediate for the preparation of agrochemicals, pharmaceuticals and other organic compounds. It is used as a precursor and reacts with sulfamethoxazole to prepare Schiff’s base, 4-[(4-Bromo-benzylidene)-amino]-N-(5-methyl-isoxazol-3-yl)-benzene sulfonamide, which can be used for the photo stability of polyvinyl chloride (PVC). It plays an important role in palladium-catalyzed mono- and bis-arylation of 3,4-(ethylenedioxy)thiophenes. It is employed in a cross-coupling study with potassium vinyltrifluoroborate.
Solubility
Soluble in ethanol and benzene. Insoluble in water.
Notes
Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong bases, strong oxidizing agents and strong reducing agents.
RUO – Research Use Only
General References:
- Huang, X.; Peng, L.; Gu, F. L.; Zhang, R. Theoretical study on catalyzed selective photoreduction mechanism for 4-bromobenzaldehyde in two different solvents. Phys. Chem. Chem. Phys. 2015, 17 (30), 19997-20005.
- Climent, C.; Alemany, P.; Lee, D.; Kim, J.; Casanova, D. Optical Properties of 4-Bromobenzaldehyde Derivatives in Chloroform Solution. J. Phys. Chem. A 2014, 118 (34), 6914-6921.