Search Thermo Fisher Scientific
Thermo Scientific Chemicals
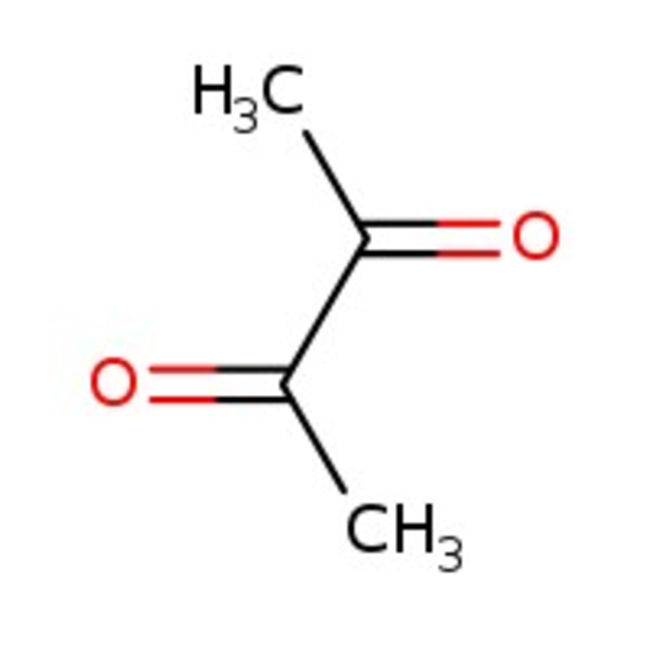
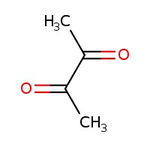
2,3-Butanedione, 99%
CAS: 431-03-8 | C4H6O2 | 86.09 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14217.09 | 10 g |
Catalog number ALFA14217.09
Price (MYR)
314.00
EA
Quantity:
10 g
Price (MYR)
314.00
EA
Specifications
Chemical Name or Material2,3-Butanedione
CAS431-03-8
Health Hazard 1H225-H302-H315-H317-H318-H331-H373
Health Hazard 2GHS H Statement
H225-H302-H332-H315-H319
Highly flammable liquid and vapour.
Harmful if swallowed.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
H225-H302-H332-H315-H319
Highly flammable liquid and vapour.
Harmful if swallowed.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P272-P280-P301+P312-P303+P361+P353-P304+P340-P305+P351+P338-P310-P311-P314-P330-P333+P313-P363-P370+P378q-P501c
View more
2,3-Butanedione is used to form triazine and pteridine ring systems by cyclocondensation with amines. It is also used to inactivate aminopeptidase-N and acts as a precursor to alpha-diones. It finds use in alcoholic beverages, foods, to modify arginyl residues in proteins and to give a buttery flavor to microwave popcorns.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,3-Butanedione is used to form triazine and pteridine ring systems by cyclocondensation with amines. It is also used to inactivate aminopeptidase-N and acts as a precursor to alpha-diones. It finds use in alcoholic beverages, foods, to modify arginyl residues in proteins and to give a buttery flavor to microwave popcorns.
Solubility
Miscible with water.
Notes
Store in a cool place. Incompatible with acids, strong bases, metals, reducing agents and oxidizing agents.
2,3-Butanedione is used to form triazine and pteridine ring systems by cyclocondensation with amines. It is also used to inactivate aminopeptidase-N and acts as a precursor to alpha-diones. It finds use in alcoholic beverages, foods, to modify arginyl residues in proteins and to give a buttery flavor to microwave popcorns.
Solubility
Miscible with water.
Notes
Store in a cool place. Incompatible with acids, strong bases, metals, reducing agents and oxidizing agents.
RUO – Research Use Only
General References:
- Sensitizer for the photochemical epoxidation of alkenes: J. Am. Chem. Soc., 98, 4193 (1976).
- Like 1,2-Cyclohexanedione, A14401, has been used by Ley's group in combination with trimethyl orthoformate and camphorsulfonic acid for the protection of trans-1,2-diols as cyclic diacetals. The method is particularly applicable to the carbohydrate field: Synlett, 793 (1996); J. Chem. Soc., Perkin 1, 2023 (1997). See also 9,10-Phenanthrenequinone, A11762.
- El-Sayed, A. E.; Al-Fulaij, O. A.; Elaasar, A. A.; El-Defrawy, M. M.; El-Asmy, A. A. Spectroscopic characterization and biological activity of dihydrazone transition metal complexes: Crystal structure of 2, 3-butanedione bis (isonicotinylhydrazone). Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 135, 211-218.
- Mikhailov, O. V.; Chachkov, D. V. Molecular structures of asymmetric (555) macrotricyclic chelates formed in 3d metal ion-ethanedithioamide-hydrazinomethanethioamide-2, 3-butanedione quaternary systems. Russ. J. Inorg. Chem. 2015, 60 (2), 187-193.