Search Thermo Fisher Scientific
Thermo Scientific Chemicals
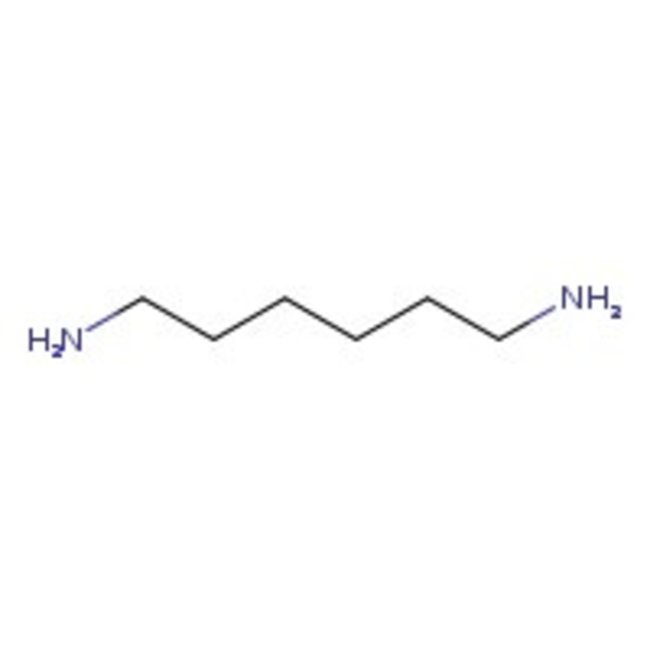
1,6-Diaminohexane, 98+%
CAS: 124-09-4 | C6H16N2 | 116.208 g/mol
Catalog number ALFA14212.36
Price (MYR)
567.00
EA
Quantity:
500 g
Price (MYR)
567.00
EA
Specifications
Chemical Name or Material1,6-Diaminohexane
CAS124-09-4
Health Hazard 1H227-H302+H312-H314-H335
Health Hazard 2GHS H Statement
H314-H318-H302-H312-H335
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
Harmful in contact with skin.
May cause respiratory irritation.
H314-H318-H302-H312-H335
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
Harmful in contact with skin.
May cause respiratory irritation.
Health Hazard 3P210-P235-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
1,6-Diaminohexane is mainly used as a monomer to make nylon 6-6. Its derivative hexamethylene diisocyanate (HDI) is used in the production of polyurethane. It acts as a cross-linking agent in epoxy resins. Other applications include coatings, lubricants and water treatment products.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,6-Diaminohexane is mainly used as a monomer to make nylon 6-6. Its derivative hexamethylene diisocyanate (HDI) is used in the production of polyurethane. It acts as a cross-linking agent in epoxy resins. Other applications include coatings, lubricants and water treatment products.
Solubility
Soluble in water. Slightly soluble in ether, alcohol and benzene.
Notes
Air sensitive and hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with acids, strong oxidizing agents, acid chlorides, carbon dioxide and acid anhydrides.
1,6-Diaminohexane is mainly used as a monomer to make nylon 6-6. Its derivative hexamethylene diisocyanate (HDI) is used in the production of polyurethane. It acts as a cross-linking agent in epoxy resins. Other applications include coatings, lubricants and water treatment products.
Solubility
Soluble in water. Slightly soluble in ether, alcohol and benzene.
Notes
Air sensitive and hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with acids, strong oxidizing agents, acid chlorides, carbon dioxide and acid anhydrides.
RUO – Research Use Only
General References:
- Machida, H.; Yamada, H.; Fujioka, Y.; Yamamoto, S. CO2 Solubility Measurements and Modeling for Tertiary Diamines. J. Chem. Eng. Data 2015, 60 (3), 814-820.
- Jong, T.; Bradley, M. Flow-Mediated Synthesis of Boc, Fmoc, and DdivMonoprotected Diamines. Org. Lett. 2015, 17 (3), 422-425.