Search Thermo Fisher Scientific
Thermo Scientific Chemicals
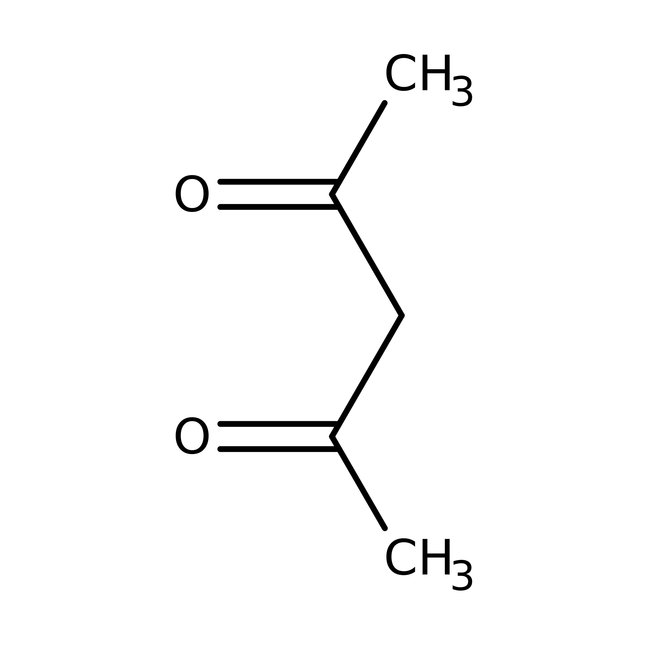
2,4-Pentanedione, 99%
CAS: 123-54-6 | C5H8O2 | 100.12 g/mol
Catalog number ALFA14117.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or Material2, 4-Pentanedione
CAS123-54-6
Health Hazard 1H226-H302-H311+H331
Health Hazard 2GHS H Statement
H311-H331-H226-H302
H311-H331-H226-H302
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P270-P271-P280-P301+P312-P303+P361+P353-P304+P340-P311-P312-P330-P363-P370+P378q-P501c
View more
2,4-Pentanedione is used as a precursor to acetylacetonate, a common bidentate and a raw material for the synthesis of heterocyclic compounds. It is used in pharmaceuticals, organic intermediates for solvents, analytical reagents, tungsten and molybdenum in aluminum extraction agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,4-Pentanedione is used as a precursor to acetylacetonate, a common bidentate and a raw material for the synthesis of heterocyclic compounds. It is used in pharmaceuticals, organic intermediates for solvents, analytical reagents, tungsten and molybdenum in aluminum extraction agent.
Solubility
It is readily miscible with water.
Notes
Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides.
2,4-Pentanedione is used as a precursor to acetylacetonate, a common bidentate and a raw material for the synthesis of heterocyclic compounds. It is used in pharmaceuticals, organic intermediates for solvents, analytical reagents, tungsten and molybdenum in aluminum extraction agent.
Solubility
It is readily miscible with water.
Notes
Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides.
RUO – Research Use Only
General References:
- A simple procedure for the synthesis of methyl ketones involves alkylation at the 3-position with an alkyl halide using K2CO3 in EtOH, with in situ acetyl cleavage: Org. Synth. Coll., 5, 767 (1973). Knoevenagel condensation with aldehydes in THF in the presence of K2CO3 also occurs with acetyl cleavage, providing a simple route to alkenyl ketones: Chem. Lett., 1325 (1978). With K2CO3 and TBAB, selective one-pot monoalkylation or dialkylation with different alkyl groups can be achieved: Synthesis, 688 (1989). 3-Arylation with aryl bromides takes place in the presence of Cu(I) or Cu(II) salts in DMF: Chem. Lett., 597 (1982); Org. Synth. Coll., 6, 36 (1988).
- Strong bases, e.g. NaNH2, form the dianion which reacts with electrophiles, e.g. alkyl halides, esters, acyl halides, Michael acceptors, etc., at the less-stabilized terminal carbon: J. Org. Chem., 26, 1716 (1961); Tetrahedron Lett., 357 (1970); J. Org. Chem., 39, 2289 (1974); Org. Synth. Coll., 5, 848 (1973). For arylation at the 1-position, see Diphenyl iodonium chloride, A15149. For reviews of dimetallation, see: Org. React., 17, 155 (1969); Synthesis, 509 (1977).
- Freeman, F. Mechanisms of Reactions of Sulfur Hydride Hydroxide: Tautomerism, Condensations, and C-Sulfenylation and O-Sulfenylation of 2,4-Pentanedione. J. Phys. Chem. A 2015, 119 (14), 3500-3517.
- Freeman, F. Mechanisms of Reactions of Sulfur Hydride Hydroxide: Tautomerism, Condensations, and C-Sulfenylation and O-Sulfenylation of 2, 4-Pentanedione. J. Phys. Chem. A 2015, 199 (14), 3500-3517.