Search Thermo Fisher Scientific
Thermo Scientific Chemicals
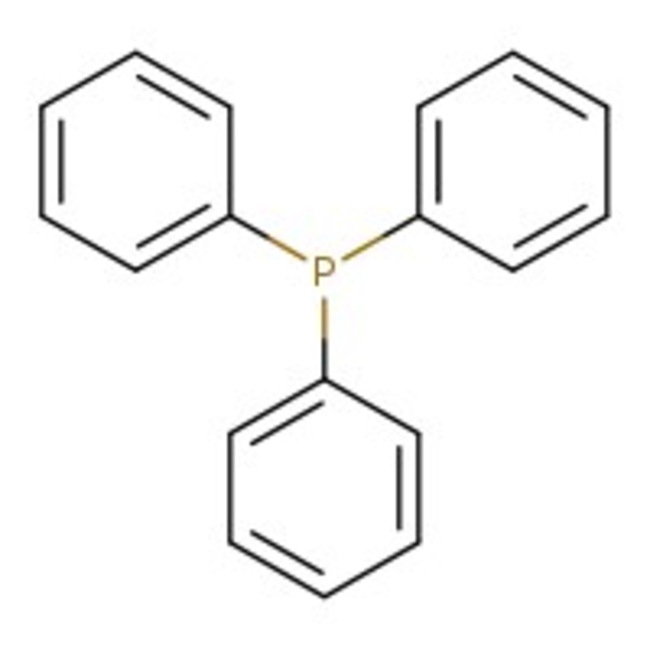
Triphenylphosphine, flake, 99%
CAS: 603-35-0 | C18H15P | 262.29 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14089.0B | 1000 g |
Catalog number ALFA14089.0B
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1000 g
Specifications
Chemical Name or MaterialTriphenylphosphine
CAS603-35-0
Health Hazard 1H302-H317-H373
Health Hazard 2GHS H Statement
H373-H302-H317
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
May cause an allergic skin reaction.
H373-H302-H317
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
May cause an allergic skin reaction.
Health Hazard 3P260-P264b-P270-P272-P280g-P301+P312-P302+P352-P314-P330-P333+P313-P363-P501c
View more
In the synthesis of organic compounds, phosphonium salts and other phosphorus compounds, and as a polymerization initiatorTriphenylphosphine is used in the synthesis of organic compounds due to its nucleophilicity and its reducing character. It is involved in the synthesis of biaryl compounds, phosphonium salts and other phosphorus compounds. As a reducing agent, it is used to prepare aromatic amines from the corresponding aromatic N-oxides. The anionic phosphine is usually isolated as the trisodium salt, which reacts with rhodium to form a complex that finds use in industrial hydroformylation reactions. It is also used to prepare Wilkinson's catalyst, RhCl(PPh3)3 useful to catalyze the hydrogenation of alkenes and tetrakis(triphenylphosphine)palladium(0) that is widely used to catalyse C-C coupling reactions in organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
In the synthesis of organic compounds, phosphonium salts and other phosphorus compounds, and as a polymerization initiatorTriphenylphosphine is used in the synthesis of organic compounds due to its nucleophilicity and its reducing character. It is involved in the synthesis of biaryl compounds, phosphonium salts and other phosphorus compounds. As a reducing agent, it is used to prepare aromatic amines from the corresponding aromatic N-oxides. The anionic phosphine is usually isolated as the trisodium salt, which reacts with rhodium to form a complex that finds use in industrial hydroformylation reactions. It is also used to prepare Wilkinson′s catalyst, RhCl(PPh3)3 useful to catalyze the hydrogenation of alkenes and tetrakis(triphenylphosphine)palladium(0) that is widely used to catalyse C-C coupling reactions in organic synthesis.
Notes
Incompatible with oxidizing agents and acids.
In the synthesis of organic compounds, phosphonium salts and other phosphorus compounds, and as a polymerization initiatorTriphenylphosphine is used in the synthesis of organic compounds due to its nucleophilicity and its reducing character. It is involved in the synthesis of biaryl compounds, phosphonium salts and other phosphorus compounds. As a reducing agent, it is used to prepare aromatic amines from the corresponding aromatic N-oxides. The anionic phosphine is usually isolated as the trisodium salt, which reacts with rhodium to form a complex that finds use in industrial hydroformylation reactions. It is also used to prepare Wilkinson′s catalyst, RhCl(PPh3)3 useful to catalyze the hydrogenation of alkenes and tetrakis(triphenylphosphine)palladium(0) that is widely used to catalyse C-C coupling reactions in organic synthesis.
Notes
Incompatible with oxidizing agents and acids.
RUO – Research Use Only
General References:
- Lenstra, D. C.; Rutjes, F. P.; Mecinovic, J. Triphenylphosphine-catalysed amide bond formation between carboxylic acids and amines. Chem. Commun. 2014, 50 (43), 5763-5766.
- Maleki, A.; Rahimi, R.; Maleki, S.; Hamidi, N. Synthesis and characterization of magnetic bromochromate hybrid nanomaterials with triphenylphosphine surface-modified iron oxide nanoparticles and their catalytic application in multicomponent reactions. RSC Adv. 2014, 4 (56), 29765-29771.