Search Thermo Fisher Scientific
Thermo Scientific Chemicals
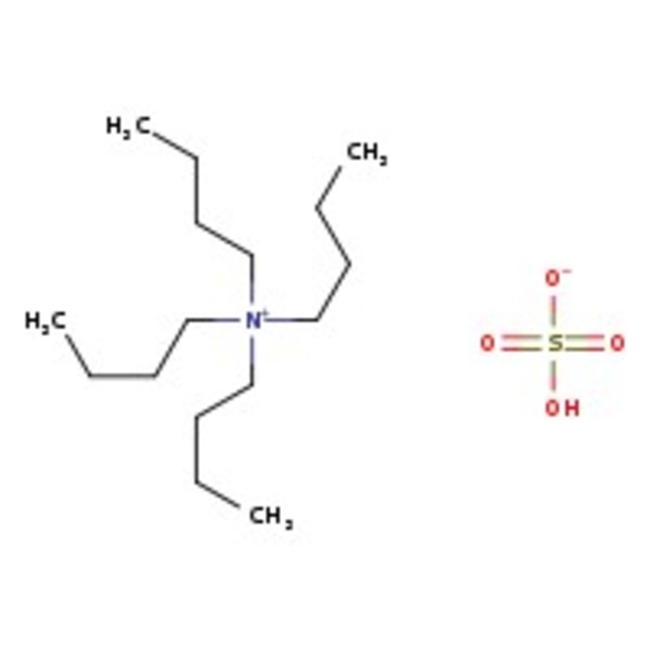
Tetra-n-butylammonium hydrogen sulfate, 97%
CAS: 32503-27-8 | C16H37NO4S | 339.54 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14047.30 | 250 g |
Catalog number ALFA14047.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialTetra-n-butylammonium hydrogen sulfate
CAS32503-27-8
Health Hazard 1H302-H315-H319-H335
Health Hazard 2GHS H Statement
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
Phase transfer catalyst Tetra-n-butylammonium hydrogen sulfate shows antibacterial properties due to the presence of the quaternary amine and the counter ion. It serves as a phase-transfer catalyst, surface-active agent, solvent, intermediate, active ingredient for conditioners, antistatic agent, detergent sanitizers, softener for textiles and paper products, emulsifying agents and pigment dispersers. It is used as an efficient catalyst in the N-alkylation reactions of benzanilides, cyclization of beta-amino acids to beta-lactams, conversion of nitriles to amides, dehydrohalogenation of aryl 2-haloethyl ethers to give vinyl ethers and in the oxidation of alcohols.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phase transfer catalyst Tetra-n-butylammonium hydrogen sulfate shows antibacterial properties due to the presence of the quaternary amine and the counter ion. It serves as a phase-transfer catalyst, surface-active agent, solvent, intermediate, active ingredient for conditioners, antistatic agent, detergent sanitizers, softener for textiles and paper products, emulsifying agents and pigment dispersers. It is used as an efficient catalyst in the N-alkylation reactions of benzanilides, cyclization of beta-amino acids to beta-lactams, conversion of nitriles to amides, dehydrohalogenation of aryl 2-haloethyl ethers to give vinyl ethers and in the oxidation of alcohols.
Solubility
Soluble in water.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
Phase transfer catalyst Tetra-n-butylammonium hydrogen sulfate shows antibacterial properties due to the presence of the quaternary amine and the counter ion. It serves as a phase-transfer catalyst, surface-active agent, solvent, intermediate, active ingredient for conditioners, antistatic agent, detergent sanitizers, softener for textiles and paper products, emulsifying agents and pigment dispersers. It is used as an efficient catalyst in the N-alkylation reactions of benzanilides, cyclization of beta-amino acids to beta-lactams, conversion of nitriles to amides, dehydrohalogenation of aryl 2-haloethyl ethers to give vinyl ethers and in the oxidation of alcohols.
Solubility
Soluble in water.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Widely used phase-transfer catalyst; see Appendix 2. Some illustrative applications are given below:
- Benzanilides have been N-alkylated in the presence of NaOH and K2CO3: Synth. Commun., 18, 2011 (1988).
- It was the most effective catalyst studied for the cyclization of ß-amino acids to ß-lactams by methanesulfonyl chloride and KHCO3: Chem. Lett., 443 (1981).
- In the dehydrohalogenation of aryl 2-haloethyl ethers to give vinyl ethers, this catalyst was found much more effective than the corresponding halides or benzyltriethylammonium salts: Synthesis, 688 (1979).
- For use in the permanganate oxidation of benzylic positions, see Potassium permanganate, A12170.
- The oxidation of primary alcohols to aldehydes by potassium chromate under phase-transfer conditions is better for higher homologues, where solubility in the aqueous phase is limited: Synthesis, 134 (1979). For phase-transfer catalyzed dichromate oxidation of secondary alcohols to ketones, see: Tetrahedron Lett., 1601 (1978).
- For phase-transfer catalyzed conversion of nitriles to amides by alkaline H2O2, see: Synthesis, 243 (1980).
- Pandian, T. S.; Choi, Y.; Srinivasadesikan, V.; Lin, M. C.; Kang, J. A dihydrogen phosphate selective anion receptor based on acylhydrazone and pyrazole. New J. Chem. 2015, 39 (1), 650-658.
- Taguchi, H.; Yanagisawa, D.; Morikawa, S.; Hirao, K.; Shirai, N.; Tooyama, I. Synthesis and Tautomerism of Curcumin Derivatives and Related Compounds. Aust. J. Chem. 2015, 68 (2), 224-229.