Search Thermo Fisher Scientific
Thermo Scientific Chemicals
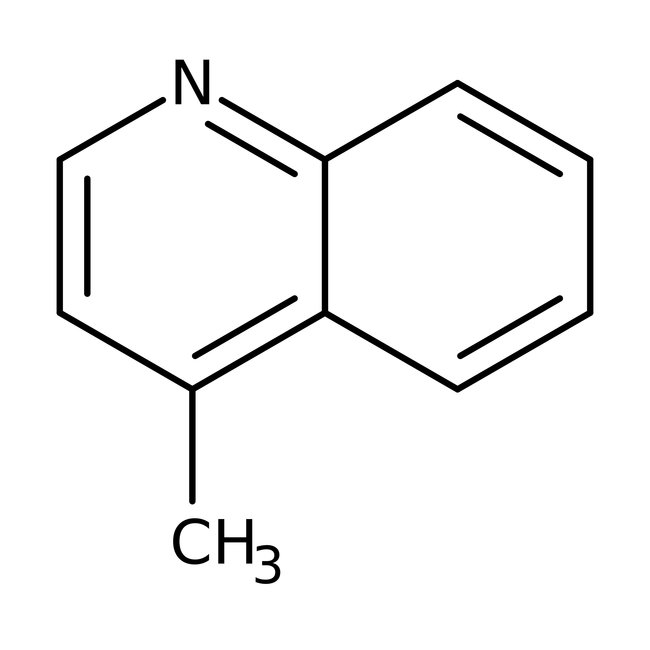
Lepidine, 97%
CAS: 491-35-0 | C10H9N | 143.189 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14040.18 | 50 g |
Catalog number ALFA14040.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Specifications
Chemical Name or MaterialLepidine
CAS491-35-0
Health Hazard 1H315-H319-H335-H341
Health Hazard 2GHS H Statement
H341-H315-H319
Suspected of causing genetic defects.
Causes skin irritation.
Causes serious eye irritation.
H341-H315-H319
Suspected of causing genetic defects.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P201-P202-P261-P264b-P271-P280i-P281-P302+P352-P304+P340-P305+P351+P338-P308+P313-P332+P313-P362-P501c
View more
Lepidine is used in the preparation of certain dyes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Lepidine is used in the preparation of certain dyes.
Solubility
Slightly soluble in water.
Notes
Light sensitive. Incompatible with oxidizing agents. Incompatible with oxidizing agents.
Lepidine is used in the preparation of certain dyes.
Solubility
Slightly soluble in water.
Notes
Light sensitive. Incompatible with oxidizing agents. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- K.R.Justin Thomas; Marappan Velusamy; Jiann T.Lin ; Chin-Hsiung Chien; Yu-Tai Tao; Yuh S.Wen; Ya-Hui Hu; Pi-Tai Chou. Efficient red-emitting cyclometalated iridium(III) complexes containing lepidine-based ligands. Inorg. Chem. 2005, 44,(16), 5677-5685
- Martin J.Weiss; Charles R.Hauser. The acylation and carbethoxylation of quinaldine, lepidine and α-picoline using sodium amide or potassium amide. J. Am. Chem. Soc. 1949, 71,(6), 2023-2026.
- Lepidine and similar compounds can be alkylated at the 2-position by free radicals generated by the action of t-butyl hydroperoxide and a salt of Fe(II) on a primary or secondary alkyl iodide: Acta Chem. Scand., 43, 995 (1989). Similarly, 2-formylation can be achieved in high yield using 1,3,5-trioxane in the presence of TFA, t-BuOOH and FeSO4 in acetonitrile: J. Org. Chem., 51, 536 (1986).