Search Thermo Fisher Scientific
Thermo Scientific Chemicals
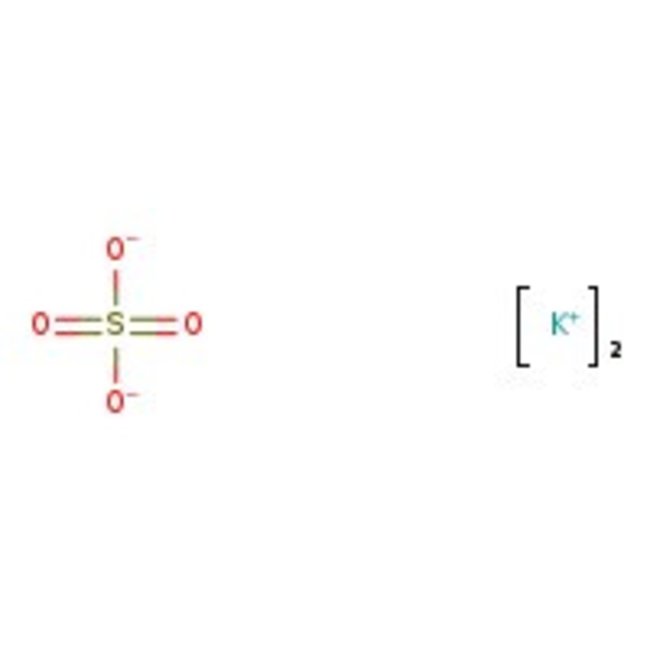
Potassium Sulfate, 99%
CAS: 7778-80-5 | K2O4S | 174.25 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13975.0B | 1000 g |
Catalog number ALFA13975.0B
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1000 g
Specifications
Boiling Point1689°C
Chemical Name or MaterialPotassium sulfate
Melting Point1,069°C
CAS7778-80-5
Recommended StorageAmbient temperatures
View more
Potassium sulfate is used in the production of fertilizers (i.e. providing potassium and sulfur), potassium alum, potassium carbonate and glass. In analytical chemistry, it is used in the determination of nitrogen by the Kjeldahl method and protein in foodstuffs. Also, it is used in the electrostatic effects on pKa values of amino acid residues in the staphylococcal nuclease. It is used in the separation of protein through electrophoresis and also serves as a laboratory reagent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Potassium sulfate is used in the production of fertilizers (i.e. providing potassium and sulfur), potassium alum, potassium carbonate and glass. In analytical chemistry, it is used in the determination of nitrogen by the Kjeldahl method and protein in foodstuffs. Also, it is used in the electrostatic effects on pKa values of amino acid residues in the staphylococcal nuclease. It is used in the separation of protein through electrophoresis and also serves as a laboratory reagent.
Solubility
Soluble in water and glycerol. Insoluble in alcohol, acetate and carbon disulfide.
Notes
Incompatible with strong oxidizing agents.
Potassium sulfate is used in the production of fertilizers (i.e. providing potassium and sulfur), potassium alum, potassium carbonate and glass. In analytical chemistry, it is used in the determination of nitrogen by the Kjeldahl method and protein in foodstuffs. Also, it is used in the electrostatic effects on pKa values of amino acid residues in the staphylococcal nuclease. It is used in the separation of protein through electrophoresis and also serves as a laboratory reagent.
Solubility
Soluble in water and glycerol. Insoluble in alcohol, acetate and carbon disulfide.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Almeelbi, T.; Bezbaruah, A. Aqueous phosphate removal using nanoscale zero-valent iron. J. Nanopart. Res. 2012, 14 (7), 1-14.
- West, M. E.; Mauer, L. J. Development of an integrated approach for the stability testing of flavonoids and ascorbic acid in powders. Food Chem. 2011, 129 (1), 51-58.