Search Thermo Fisher Scientific
Thermo Scientific Chemicals
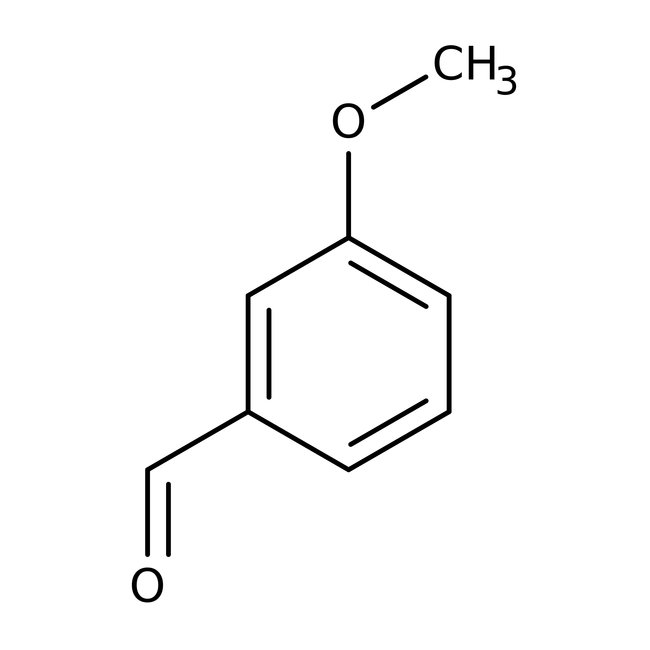
3-Methoxybenzaldehyde, 98%
CAS: 591-31-1 | C8H8O2 | 136.15 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13962.14 | 25 g |
Catalog number ALFA13962.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material3-Methoxybenzaldehyde
CAS591-31-1
Health Hazard 1H315-H319
Health Hazard 2GHS H Statement
H315-H319
Causes skin irritation.
Causes serious eye irritation.
H315-H319
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P264b-P280-P302+P352-P305+P351+P338-P332+P313-P362
View more
3-Methoxybenzaldehyde is used to prepare 3-(3-methoxy-phenyl)-1-phenyl-propenone by reaction with benzldehyde. It is used as an eluent for mono-13C isotopomers of vanillin in normal phase silica gel chromatography. It acts as an inhibitor of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3-Methoxybenzaldehyde is used to prepare 3-(3-methoxy-phenyl)-1-phenyl-propenone by reaction with benzldehyde. It is used as an eluent for mono-13C isotopomers of vanillin in normal phase silica gel chromatography. It acts as an inhibitor of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism.
Solubility
Slightly soluble in water. Soluble in glacial acetic acid, alcohol, chloroform, ether, carbon disulfide, methanol, aqueous solutions of alkali hydroxides.
Notes
Air sensitive. Incompatible with strong bases, strong oxidizing agents and strong reducing agents.
3-Methoxybenzaldehyde is used to prepare 3-(3-methoxy-phenyl)-1-phenyl-propenone by reaction with benzldehyde. It is used as an eluent for mono-13C isotopomers of vanillin in normal phase silica gel chromatography. It acts as an inhibitor of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism.
Solubility
Slightly soluble in water. Soluble in glacial acetic acid, alcohol, chloroform, ether, carbon disulfide, methanol, aqueous solutions of alkali hydroxides.
Notes
Air sensitive. Incompatible with strong bases, strong oxidizing agents and strong reducing agents.
RUO – Research Use Only
General References:
- Lithiation and methylation normally gives a mixture of 2-, 4- and 6-methyl derivatives. Systematic study has found that using PhLi and trimethylethylenediamine increases the degree of 2-lithiation to 96%: J. Org. Chem., 54, 3730 (1989).
- Bhowmick, D.; Srivastava, S.; D’Silva, P.; Mugesh, G. Highly Efficient Glutathione Peroxidase and Peroxiredoxin Mimetics Protect Mammalian Cells against Oxidative Damage. Angew. Chem. 2015, 127 (29), 8569-8573.
- Marri, G.; Reddy, J. S.; Ruiz, J.; Das, S.; Gree, R. Stereoselective TiCl4-Mediated Aldol Reactions Starting from Acylsilanes. Eur. J. Org. Chem. 2015, 2015 (4), 840-846.