Search Thermo Fisher Scientific
Thermo Scientific Chemicals
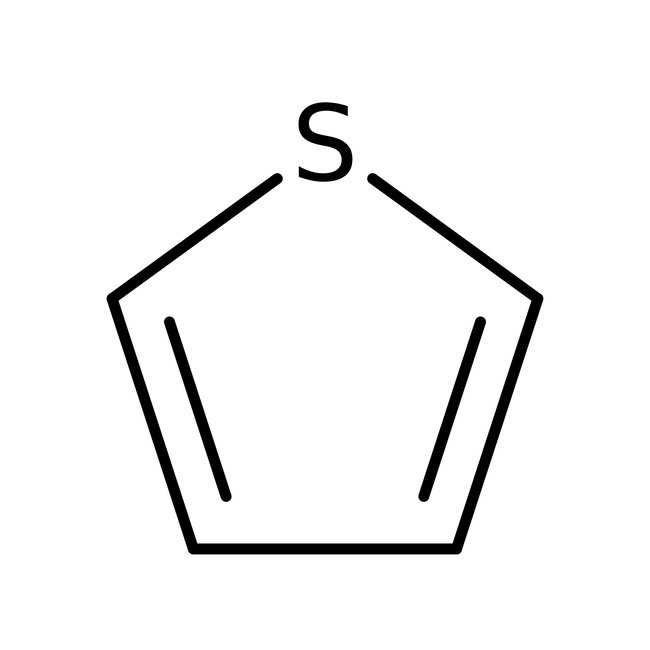
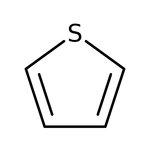
Thiophene, 99%
CAS: 110-02-1 | C4H4S | 84.14 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13941.36 | 500 g |
Catalog number ALFA13941.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialThiophene
CAS110-02-1
Health Hazard 1H225-H302-H319
Health Hazard 2GHS H Statement
H225-H331-H302-H312-H315-H319
Highly flammable liquid and vapour.
Toxic if inhaled.
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
H225-H331-H302-H312-H315-H319
Highly flammable liquid and vapour.
Toxic if inhaled.
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P210-P233-P240-P241-P242-P243-P264b-P270-P280-P301+P312-P303+P361+P353-P305+P351+P338-P330-P370+P378q-P501c
View more
Thiophene is an important building block in dyes, agrochemicals and pharmaceuticals synthesis. It is involved in the chloroalkylation reactions in 2,5-positions. It is also used to prepare butane by reduction with raney nickel, 2-vinylthiophene, dithienyl, and 2-halo thiophenes by reacting with halogens.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Thiophene is an important building block in dyes, agrochemicals and pharmaceuticals synthesis. It is involved in the chloroalkylation reactions in 2,5-positions. It is also used to prepare butane by reduction with raney nickel, 2-vinylthiophene, dithienyl, and 2-halo thiophenes by reacting with halogens.
Solubility
Miscible with ethyl alcohol, diethyl ether and other organic solvents. Immiscible with water.
Notes
Incompatible with strong oxidizing agents and strong acids.
Thiophene is an important building block in dyes, agrochemicals and pharmaceuticals synthesis. It is involved in the chloroalkylation reactions in 2,5-positions. It is also used to prepare butane by reduction with raney nickel, 2-vinylthiophene, dithienyl, and 2-halo thiophenes by reacting with halogens.
Solubility
Miscible with ethyl alcohol, diethyl ether and other organic solvents. Immiscible with water.
Notes
Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Metallation at the 2-position occurs readily with n-BuLi in ether (not readily in benzene). The 2,5-dilithio-derivative can be prepared in the presence of TMEDA: J. Chem. Soc., Perkin 1, 887 (1977), or with the superbasic combination of n-BuLi with KO-t-Bu: Synthesis, 316 (1988).
- Smith, Z. C.; Meyer, D. M.; Simon, M. G.; Staii, C.; Shukla, D.; Thomas III, S. W. Thiophene-Based Conjugated Polymers with Photolabile Solubilizing Side Chains. Macromolecules 2015, 48 (4), 959-966.
- Ghosh, T.; Gopal, A.; Saeki, A.; Seki, S.; Nair, V. C. p-Polarity of thiophene oligomers in photovoltaic cells: role of molecular vs. supramolecular properties. Phys. Chem. Chem. Phys. 2015, 17 (16), 10630-10639.