Search Thermo Fisher Scientific
Thermo Scientific Chemicals
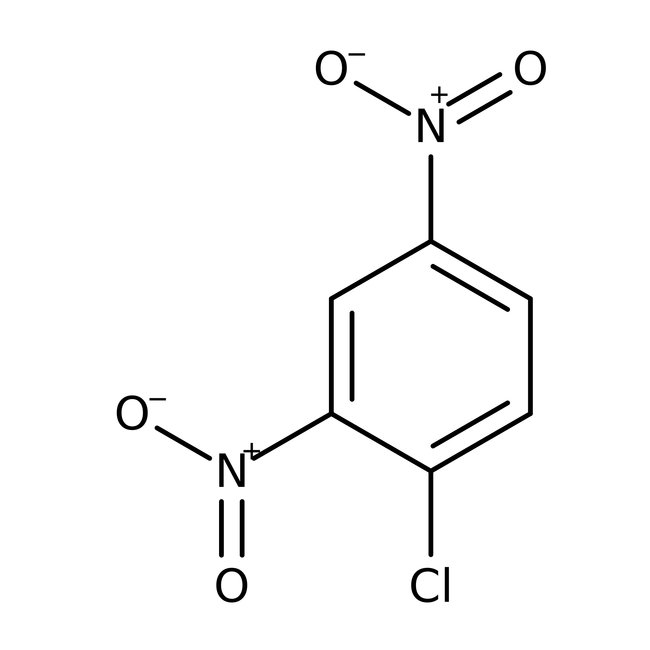
1-Chloro-2,4-dinitrobenzene, 98%
CAS: 97-00-7 | C6H3ClN2O4 | 202.55 g/mol
Catalog number ALFA13774.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or Material1-Chloro-2,4-dinitrobenzene
CAS97-00-7
Health Hazard 1H302-H310-H315-H317-H318
Health Hazard 2GHS H Statement
H301-H311-H331-H373
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
May cause damage to organs through prolonged or repeated exposure.
H301-H311-H331-H373
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
May cause damage to organs through prolonged or repeated exposure.
Health Hazard 3P261-P262-P264b-P270-P272-P280-P301+P312-P302+P350-P305+P351+P338-P310-P330-P333+P313-P361-P363-P501c
View more
1-Chloro-2,4-dinitrobenzene is used as a reagent for the detection and determination of pyridine compounds. It has been used as alkylating agent to evaluate the depletion of intracellular erythrocyte glutathione (GSH). It is an irreversible inhibitor of human thioredoxin reductase.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Chloro-2,4-dinitrobenzene is used as a reagent for the detection and determination of pyridine compounds. It has been used as alkylating agent to evaluate the depletion of intracellular erythrocyte glutathione (GSH). It is an irreversible inhibitor of human thioredoxin reductase.
Solubility
Soluble in chloroform, ether, hot alcohol, and benzene. Insoluble in water.
Notes
Incompatible with strong oxidizing agents, ammonia. Reacts with hydrazine hydrate.
1-Chloro-2,4-dinitrobenzene is used as a reagent for the detection and determination of pyridine compounds. It has been used as alkylating agent to evaluate the depletion of intracellular erythrocyte glutathione (GSH). It is an irreversible inhibitor of human thioredoxin reductase.
Solubility
Soluble in chloroform, ether, hot alcohol, and benzene. Insoluble in water.
Notes
Incompatible with strong oxidizing agents, ammonia. Reacts with hydrazine hydrate.
RUO – Research Use Only
General References:
- Elias S. J. Arner; Mikael Bjornstedt and Arne Holmgren. 1-Chloro-2,4-dinitrobenzene Is an Irreversible Inhibitor of Human Thioredoxin Reductase. Loss of thioredoxin disulfide reductase activity is accompanied by a large increase in nadph oxidase activity.Journal of Biological Chemistry.1995, 270 3479-3482.
- YC Awasthi; HS Garg; DD Dao; CA Partridge and SK Srivastava. Enzymatic conjugation of erythrocyte glutathione with 1-chloro-2,4-dinitrobenzene: the fate of glutathione conjugate in erythrocytes and the effect of glutathione depletion on hemoglobin. bloodjournal.hematologylibrary.1981, 58 733-738.
- The doubly-activated chloro-substituent is readily displaced by nucleophiles, e.g.: Ammonia/ammonium acetate to give 2,4-dinitroaniline: Org. Synth. Coll., 2, 221 (1943); or with ammonia gas in toluene in the presence of TBAB which improves the solubility of ammonia in the organic medium and accelerates the reaction: J. Chem. Soc., Chem. Commun., 1267 (1987).
- Benzyl mercaptan to give the thioether: Org. Synth. Coll., 5, 474 (1973).
- Iodide ion to give 2,4-dinitro-1-iodobenzene: Org. Synth. Coll., 5, 478 (1973). Nitrite in toluene (phase-transfer) to give 1,2,4-trinitrobenzene (caution!): J. Org. Chem., 56, 4967 (1991).