Search Thermo Fisher Scientific
Thermo Scientific Chemicals
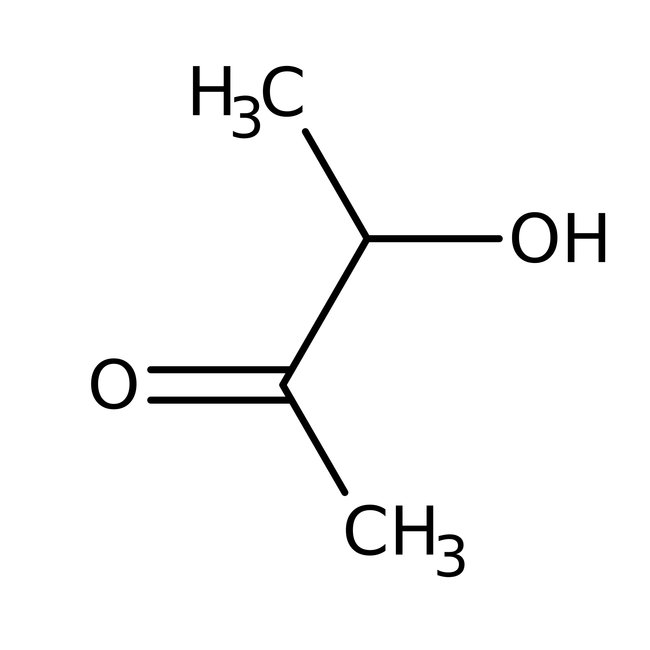
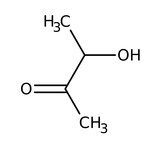
3-Hydroxy-2-butanone, monomer + dimer, 95%
CAS: 513-86-0 | C4H8O2 | 88.11 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13752.18 | 50 g |
Catalog number ALFA13752.18
Price (MYR)
428.00
EA
Quantity:
50 g
Price (MYR)
428.00
EA
Specifications
Chemical Name or Material3-Hydroxy-2-butanone
Name Notemonomer + dimer
CAS513-86-0
Health Hazard 1H226-H315-H319
Health Hazard 2GHS H Statement
H226-H315-H319
Flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
H226-H315-H319
Flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
View more
3-Hydroxy-2-butanone is a chemical used in food flavoring and fragrances. It acts as an intermediate of butanediol cycle in microorganisms. It is used as an aroma carrier in the preparation of flavors and essences.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3-Hydroxy-2-butanone is a chemical used in food flavoring and fragrances. It acts as an intermediate of butanediol cycle in microorganisms. It is used as an aroma carrier in the preparation of flavors and essences.
Solubility
Miscible with water, propylene glycol and alcohol. Slightly miscible with ether, petroleum ether. Immiscible with fatty oils and vegetable oil.
Notes
Store in cool place. Incompatible with strong oxidizing agents.
3-Hydroxy-2-butanone is a chemical used in food flavoring and fragrances. It acts as an intermediate of butanediol cycle in microorganisms. It is used as an aroma carrier in the preparation of flavors and essences.
Solubility
Miscible with water, propylene glycol and alcohol. Slightly miscible with ether, petroleum ether. Immiscible with fatty oils and vegetable oil.
Notes
Store in cool place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Solid dimer is converted to liquid monomer on melting, dissolution or distillation. Gradually reverts to dimer on standing.
- Praske, E.; Crounse, J. D.; Bates, K. H.; Kurten, T.; Kjaergaard, H. G.; Wennberg, P. O. Atmospheric Fate of Methyl Vinyl Ketone: Peroxy Radical Reactions with NO and HO2. J. Phys. Chem. A 2015, 119 (19), 4562-4572.
- Liu, J.; Liu, M.; He, C.; Song, H.; Chen, F. Effect of thermal treatment on the flavor generation from Maillard reaction of xylose and chicken peptide. LWT Food Sci. Technol. 2015, 64 (1), 316-325.