Search Thermo Fisher Scientific
Thermo Scientific Chemicals
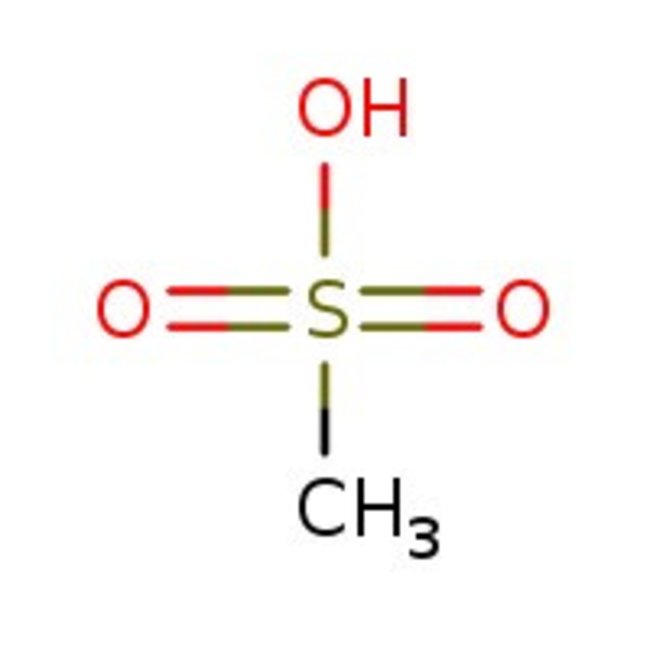
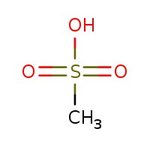
Methanesulfonic acid, 98+%
CAS: 75-75-2 | CH4O3S | 96.1 g/mol
Catalog number ALFA13565.36
Price (MYR)
578.00
EA
Quantity:
500 g
Price (MYR)
578.00
EA
Specifications
Chemical Name or MaterialMethanesulfonic acid
CAS75-75-2
Health Hazard 1H290-H302+H312-H314-H335
Health Hazard 2GHS H Statement
H314
Causes severe skin burns and eye damage.
H314
Causes severe skin burns and eye damage.
Health Hazard 3P234-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P390-P501c
View more
Methanesulfonic acid is used as a catalyst in organic reactions namely esterification, alkylation and condensation reactions due to its non- volatile nature and solubility in organic solvents. It is also involved in the production of starch esters, wax oxidate esters, benzoic acid esters, phenolic esters, or alkyl esters. It reacts with sodium borohydride in presence of polar solvent tetrahydrofuran to prepare borane-tetrahydrofuran complex. It finds application in batteries, because of its purity and chloride absence. In pharmaceutical industry, it is used for the manufacturing of active pharmaceutical ingredients like telmisartan and eprosartan. It is useful in ion chromatography and is a source of carbon and energy for some gram-negative methylotropic bacteria.It is involved in the deprotection of peptides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methanesulfonic acid is used as a catalyst in organic reactions namely esterification, alkylation and condensation reactions due to its non- volatile nature and solubility in organic solvents. It is also involved in the production of starch esters, wax oxidate esters, benzoic acid esters, phenolic esters, or alkyl esters. It reacts with sodium borohydride in presence of polar solvent tetrahydrofuran to prepare borane-tetrahydrofuran complex. It finds application in batteries, because of its purity and chloride absence. In pharmaceutical industry, it is used for the manufacturing of active pharmaceutical ingredients like telmisartan and eprosartan. It is useful in ion chromatography and is a source of carbon and energy for some gram-negative methylotropic bacteria.It is involved in the deprotection of peptides.
Notes
Moisture, light and heat sensitive. Hygroscopic. Keep container tightly closed in a dry and well-ventilated place. Incompatible with amines, strong reducing agents, ethyl vinyl ether, hydrofluoric acid, strong oxidizing agents and bases.
Methanesulfonic acid is used as a catalyst in organic reactions namely esterification, alkylation and condensation reactions due to its non- volatile nature and solubility in organic solvents. It is also involved in the production of starch esters, wax oxidate esters, benzoic acid esters, phenolic esters, or alkyl esters. It reacts with sodium borohydride in presence of polar solvent tetrahydrofuran to prepare borane-tetrahydrofuran complex. It finds application in batteries, because of its purity and chloride absence. In pharmaceutical industry, it is used for the manufacturing of active pharmaceutical ingredients like telmisartan and eprosartan. It is useful in ion chromatography and is a source of carbon and energy for some gram-negative methylotropic bacteria.It is involved in the deprotection of peptides.
Notes
Moisture, light and heat sensitive. Hygroscopic. Keep container tightly closed in a dry and well-ventilated place. Incompatible with amines, strong reducing agents, ethyl vinyl ether, hydrofluoric acid, strong oxidizing agents and bases.
RUO – Research Use Only
General References:
- Strong acid which has been used for the deprotection of peptides; see Appendix 6.
- Cleaves methyl ethers of phenols in the presence of the soft nucleophile methionine as a methyl acceptor. Polymethoxyaldehydes or ketones undergo selective cleavage of the methoxy group meta to the carbonyl: J. Chem. Soc., Perkin 1, 2288 (1977). Similarly, in combination with dimethyl sulfide, selective O-demethylation of mixed phosphate esters occurs: Synthesis, 451 (1983).
- Sandoval, A. P.; Herrera, M. F. S.; Climent, V.; Feliu, J. M. Interaction of water with methanesulfonic acid on Pt single crystal electrodes. Electrochem. Commun. 2015, 50, 47-50.
- Chen, H.; Ezell, M. J.; Arquero, K. D.; Varner, M. E.; Dawson, M. L.; Gerber, R. B.; Pitts, B. J. F. New particle formation and growth from methanesulfonic acid, trimethylamine and water. Phys. Chem. Chem. Phys. 2015, 17 (20), 13699-13709.