Search Thermo Fisher Scientific
Thermo Scientific Chemicals
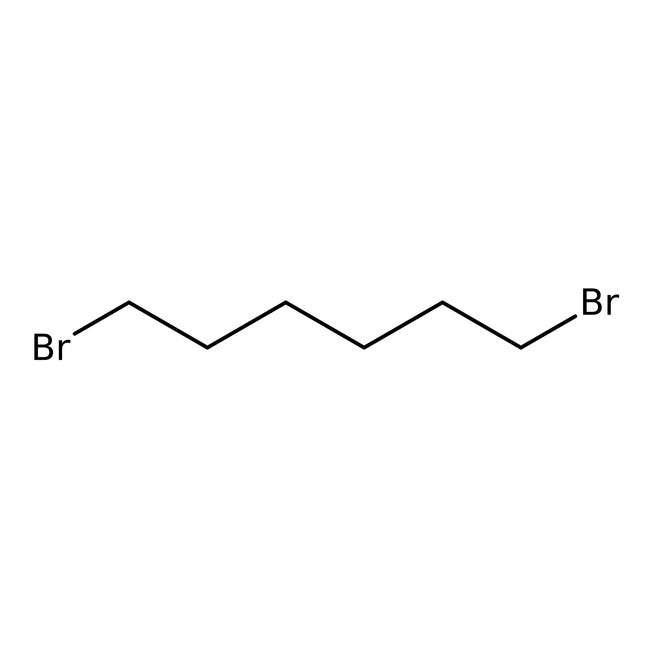
1,6-Dibromohexane, 97+%
CAS: 629-03-8 | C6H12Br2 | 243.97 g/mol
Catalog number ALFA13417.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or Material1,6-Dibromohexane
CAS629-03-8
Health Hazard 1H302-H315-H317-H319-H335
Health Hazard 2GHS H Statement
H302-H317
Harmful if swallowed.
May cause an allergic skin reaction.
H302-H317
Harmful if swallowed.
May cause an allergic skin reaction.
Health Hazard 3P261-P264b-P270-P272-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P333+P313-P363-P501c
View more
1,6-Dibromohexane is used as a reagent in the synthesis of novel benzo[b]xanthone derivatives which have a potential antitumor activity. It is also used as a cross-linker for the cross-linking of glycuronans.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,6-Dibromohexane is used as a reagent in the synthesis of novel benzo[b]xanthone derivatives which have a potential antitumor activity. It is also used as a cross-linker for the cross-linking of glycuronans.
Solubility
Miscible with ethanol, ether, benzene and chloroform. Immiscible with water.
Notes
Incompatible with strong oxidizing agents and strong bases.
1,6-Dibromohexane is used as a reagent in the synthesis of novel benzo[b]xanthone derivatives which have a potential antitumor activity. It is also used as a cross-linker for the cross-linking of glycuronans.
Solubility
Miscible with ethanol, ether, benzene and chloroform. Immiscible with water.
Notes
Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Gadgil, B.; Dmitrieva, E.; Damlin, P.; Aaritalo, T.; Kvamstrom, C. Redox reactions in a linear polyviologen derivative studied by in situ ESR/UV-vis-NIR spectroelectrochemistry. J. Solid State Electrochem. 2015, 19 (1), 77-83.
- Maeda, C.; Taniguchi, T.; Ogawa, K.; Ema, T. Bifunctional Catalysts Based on m-Phenylene-Bridged Porphyrin Dimer and Trimer Platforms: Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides. Angew. Chem. 2015, 127 (1), 136-140.