Search Thermo Fisher Scientific
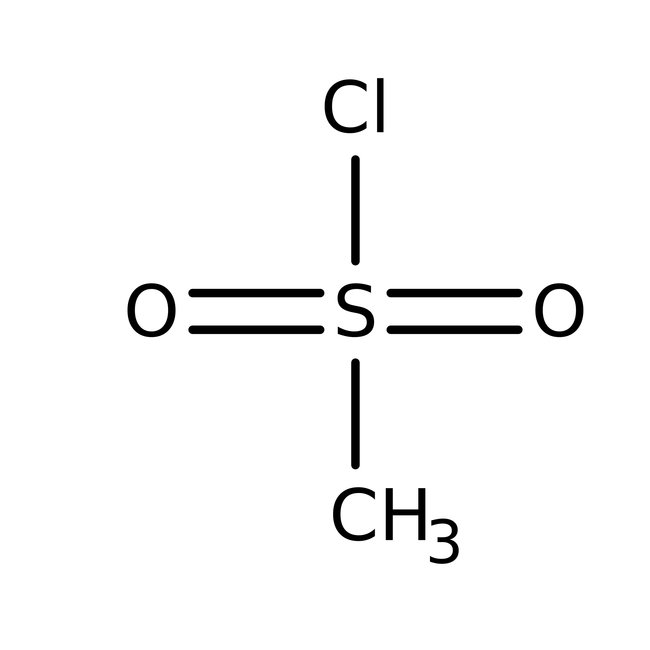
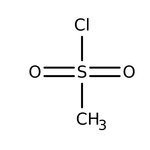
Methanesulfonyl chloride, 98%
| Catalog Number | Quantity |
|---|---|
| ALFA13383.22 | 100 g |
H300-H310-H330-H314-H318
Methanesulfonyl chloride is used as a reagent for conversion of alcohols to mesylate esters such as methanesulfonate, which is an intermediate in substitution reactions, elimination reactions, reductions, and rearrangement reactions viz. Beckmann rearrangement. It is an electrophile and acts as a source of CH3SO2+ group. It is also used to prepare beta-chloro sulfones, methanesulfonamide and heterocyclic compounds containing five membered sultones.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Methanesulfonyl chloride is used as a reagent for conversion of alcohols to mesylate esters such as methanesulfonate, which is an intermediate in substitution reactions, elimination reactions, reductions, and rearrangement reactions viz. Beckmann rearrangement. It is an electrophile and acts as a source of CH3SO2+ group. It is also used to prepare beta-chloro sulfones, methanesulfonamide and heterocyclic compounds containing five membered sultones.
Solubility
Miscible with alcohol, ether and organic solvents. Immiscible with water.
Notes
Moisture sensitive. Store in a cool place. Incompatible with strong bases, oxidizing agents and alcohols. It is a lachrymator and reacts with water.
General References:
- Reagent for conversion of alcohols to their mesylate esters. For mesylation of an acetylenic alcohol followed by displacement with an amine, see Org. Synth. Coll., 9, 46 (1998). In the presence of triethylamine, reaction occurs by way of the sulfene intermediate, which mesylates alcohols of low nucleophilicity including tert-alcohols and 2,2,2-trifluoroethanol: J. Org. Chem., 35, 3195 (1970).
- In pyridine, primary alcohols are converted to alkyl chlorides, via nucleophilic displacement by Cl- on the mesylate: Chem. Pharm. Bull., 24, 365 (1976); J. Org. Chem., 48, 657 (1983). In combination with LiCl in collidine, allylic alcohols are converted to chlorides without allylic rearrangement: J. Org. Chem., 36, 3044 (1971).
- For use in the symmetrical coupling of aryl mesylates to give biaryls, catalyzed by Dichlorobis(triphenyl phosphine) nickel(II) , 13930, see: J. Org. Chem., 60, 6895 (1995).
- Esters of hindered acids have been prepared via the mesyl mixed anhydride and reaction with the alcohol in the presence of DMAP: Synth. Commun., 12, 727 (1982).
- Zhan, N.; Palui, G.; Mattoussi, H. Preparation of compact biocompatible quantum dots using multicoordinating molecular-scale ligands based on a zwitterionic hydrophilic motif and lipoic acid anchors. Nat. Protoc 2015, 10 (6), 859-874.
- Wang, H.; Cheng, F.; Li, M.; Peng, W.; Qu, J. Reactivity and Kinetics of Vinyl Sulfone-Functionalized Self-Assembled Monolayers for Bioactive Ligand Immobilization. Langmuir 2015, 31 (11), 3413-3421.